A leading cannabis researcher went over his work on CBD and THC compounds to leverage the therapeutic potential of the endogenous cannabinoid system in the body.
Here are some observations from the Monday April 4th University of Washington Center for Cannabis Research (UW CCR) webinar on “Optimizing the Medical Properties of Cannabis.”
My top 3 takeaways:
- Nephi Stella, University of Washington Center for Cannabis Research (UW CCR) Director, provided his own background, explained the history of the center, and gave an agenda overview.
- Stella had been a prominent voice for cannabis research in policy circles, speaking about the subject to state lawmakers in April 2019 and acting as lead author of “Frameworks for Future Cannabis Research,” a legislative report authorized through a 2020 operating budget proviso and completed in December 2021.
- Stella was a panelist for the Washington State Liquor and Cannabis Board (WSLCB) deliberative dialogues on cannabis plant chemistry in June and July 2021. He went on to speak at a third dialogue on cannabis impairment hosted by agency leaders on April 27th.
- He opened the webinar noting it was the first in a series planned by the UW CCR. After identifying his position with the organization, he indicated that he was the Principal Investigator of the Stella Lab as well as leading Stella Therapeutics, Inc. and Stella Consulting LLC. He affirmed that all the research he’d be speaking to had been “published in peer-reviewed journals” (audio - 1m, video).
- Stella introduced the topic by telling attendees that he’d been involved “in the cannabinoid field for 25 years,” studying specific compounds and “how these molecules travel in our bodies, depending on the route of administration. This is the concept of pharmacokinetics.” He also researched “how the molecules actually interact with their receptor, their targets,” formally termed “pharmacodynamics.” Stella planned to focus on tetrahydrocannabinol (THC) and cannabidiol (CBD) experiments “in cells and culture, in preclinical rodent models, as well as in humans.” His lab worked to “discover new cannabinoid-based transformative therapeutics for the treatment of devastating diseases,” highlighting endeavors on “the antiepileptic properties of cannabinoids as well as the properties to directly kill brain cancers.” Stella shared his ambition to move the research “from the lab all the way to human clinical trials” (audio - 2m, video).
- As the founder of the UW CCR, Stella touched upon the center’s background, staff, and research. Established in 2017, the center brought together “the UW researchers that would be interested,” set up a board of directors, and held their “first retreat in 2019” with counterparts from Washington State University (WSU). Stella stated the group had since partnered with research institutions in California and Colorado and the university’s Addictions, Drug, and Alcohol Institute (UW ADAI). The endgame for these affiliations would be “to solidify and protect long-term policies” (audio - 3m, video).
- He described the board as “quite diverse, also, in its areas of interest” and representing varied social and scientific schools within UW.
- Over the preceding five years, UW CCR had “accumulated approximately $14 million in [National Institutes of Health] grants” as well as funding from other institutions, Stalla reported. The spending to date emphasized research focused on the effects of cannabis in adolescents, the plant’s “impairing effect,” and its impact on post-traumatic stress disorder (PTSD) along with “growing interest of the activity of CBD.”
- Learn more about the UW CCR/WSU Partnership, and the WSU Center for Cannabis Policy, Research, and Outreach (WSU CCPRO).
- Stella outlined the agenda, telling attendees his plan to make them into “experts in cannabis pharmacology” by first explaining the history and biology of the plant and its “unique medical properties.” Next, Stella said he’d discuss optimization of “therapeutic properties” of cannabinoids and the “concept of therapeutic index,” which was achieving a desired medical result with “minimal or [no] side effects.” He would also touch upon risks of cannabinoid use by teens, “progress on the therapeutic optimization of” cannabis in treating epilepsy and brain cancer, before concluding with “some challenges and future directions” in the research field (audio - 2m, video).
- Stella’s presentation went on to cover several areas including a brief history of the plant, cannabinoid bioactivity, his scientific approach, current research, and legal barriers to further study.
- A Brief History (audio - 3m, video)
- Acknowledging the long history of cannabis as a medicinal herb and fiber, Stella noted its more recent history in Europe and the U.S., when Sir William Brooke O'Shaughnessy published early medical cannabis research. He claimed that cannabis use increased “during alcohol prohibition” in the 20th century.
- Stella remarked on how the U.S. Supreme Court upheld the Schedule I controlled substance status of cannabis, meaning the government recognized no medical value from the plant, something “that’s clearly changing these days.” He noted how federal authorities began to allow more research around the medical potential of cannabinoids following an NIH panel’s recommendation in 1997. By 2017, the National Academy of Sciences published a report on the existing research around medical effects of the plant, “but they also identified some risk of cannabinoid use, chronically, at high dose which were associated with some mental health issues and some potential” substance abuse behaviors, he relayed. Defining the properties of cannabinoids, both medical and “intoxicating” impacts, was the next hurdle for experts, suggested Stella.
- Cannabinoid Bioactivity (audio - 4m, video)
- Looking at differences between THC and CBD and “what is currently accepted by the scientific community,” Stella commented that the compounds possessed similar morphology. However, scientists respected the legal distinction “between the cannabis plant and hemp” based on how much THC the plant produced, whereas there were no “legal…parameters” around how much CBD was produced by a plant. Stella thought a CBD standard in plants would “probably have to be addressed in the future.”
- Learn more about the NIH resource, The Name of Cannabis: A Short Guide for Nonbotanists.
- “Another big difference that you can see” was hydrophobic rings, meaning the molecules “were very greasy,” said Stella, and had distinct differences in “bioactivities in vivo.” He distinguished some of the effects, mentioning that THC was more psychoactive, with analgesic and appetite enhancing characteristics, in addition to impacts on brain development. He then outlined how CBD had anti-inflammatory and anti-epileptic properties, and seemed to limit the effects of THC. “A balance between CBD and THC might actually be a really interesting medical approach,” Stella added. He mentioned that side effects of CBD hadn’t been identified, though they may exist when used in high concentrations or by “vulnerable populations.”
- Stella said there was “a continuum” of bioactivity between the compounds. THC was able to assist with “sleep impairment” and stress, while also having “clear harm reduction effects” in lowering use of opioids (audio - 2m, video).
- Harms from the compound were most apparent “during embryonic development” and “adolescent brain development,” which he suggested could lead to onset of “cannabis use disorder in some vulnerable individuals.”
- CBD had clear medical potential as well, according to Stella, who identified areas of interest around using the compound in treatments for “autism and sleep.” It might also have harm reduction potential for replacing opioids used in “chronic pain,” he argued, seeing this as “a growing area of interest in our field.”
- Looking at differences between THC and CBD and “what is currently accepted by the scientific community,” Stella commented that the compounds possessed similar morphology. However, scientists respected the legal distinction “between the cannabis plant and hemp” based on how much THC the plant produced, whereas there were no “legal…parameters” around how much CBD was produced by a plant. Stella thought a CBD standard in plants would “probably have to be addressed in the future.”
- Scientific Approach (audio - 6m, video)
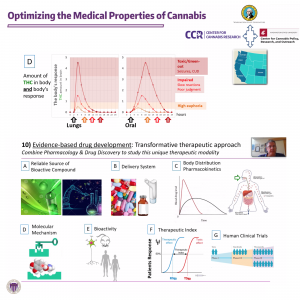
- Stella articulated a goal of studying the cannabis plant and its pharmacological aspects to make better medicines, comparing it with the development of aspirin and opioids. He believed that cannabinoids could be made to target particular enzymes in the body to lower side effects of other drugs, sharing that was “what we’re gonna try and do with cannabinoids.” Using “evidence-based drug development," Stella was working towards a "transformative therapeutic approach" for underserved patients.
- He thought that experts needed a “reliable source” of the compounds, whether that came “extracted from the plant” or via “chemical synthesis of those compounds” in a laboratory setting. After this, a delivery system for the compounds would be determined where pharmacokinetics came into play, Stella stated, typically a choice between a “rapid onset that reaches high concentrations and then is transient,” like inhalation, or a more slowly ingested oral route. At that point, pharmacodynamics would matter, since “as the cannabinoid unlocks this receptor then you change neuronal activity.”
- Established pharmacokinetics and pharmacodynamics would be used to make therapeutic indexes for cannabinoids to show what concentrations produced desired impacts or side effects, Stella communicated. The evidence-based approach could be complemented by a practice-based approach, as was happening with CBD products where surveys were giving researchers insight into increasingly available consumer products.
- Cannabis (audio - 3m, video)
- Stella explained that cannabis had been included “about ten years ago” in the American Herbal Pharmacopoeia, a publication with basic plant information. He expressed an interest in cannabis cultivar names, calling out “Foggy Mountain.” Some researchers wanted the ability to “synthesize” artificial cannabinoids which were molecularly distinct from what the plant produced, which already contained “diverse types of…phenotypes.” Stella added that other compounds like terpenes had their own bioactivity profiles.
- Pharmacokinetics (audio - 4m, video)
- Evaluating available products, Stella noted a “sharp increase” in average cannabinoid concentration both in cannabis flower, edibles, and concentrate products. He highlighted unregulated products that were so strong they could lead to “very severe cannabimimetic effects in humans.” Oral administration meant cannabinoids went through “the first step” of metabolism in the liver, while inhalation “goes to the brain directly.”
- UW ADAI researchers were leaders in a 2020 “consensus statement” on “Cannabis Concentration and Health Risks,” and published a High-Potency Cannabis website.
- In 2021, HB 1463, “Addressing serious mental health consequences of high-potency cannabis products by regulating the sale of cannabis concentrates,” proposed new restrictions on cannabis concentrates by appealing to a desire to protect youth and vulnerable populations.
- Stella reviewed typical cannabis product concentrations, routes of administration, and corresponding levels of bioactivity, making clear some levels were considered toxic. He described a phenomenon that consumers could “green out" from concentrated cannabis, which he termed “reminiscent'' of “blacking out with alcohol.”
- Evaluating available products, Stella noted a “sharp increase” in average cannabinoid concentration both in cannabis flower, edibles, and concentrate products. He highlighted unregulated products that were so strong they could lead to “very severe cannabimimetic effects in humans.” Oral administration meant cannabinoids went through “the first step” of metabolism in the liver, while inhalation “goes to the brain directly.”
- Pharmacodynamics (audio - 3m, video)
- Looking at how “the key interact with its lock to change neuronal activity,” Stella commented that THC mostly interacted with the CB1 and CB2 receptors, and CBD engaged with a receptor known as GPR55. CB1 receptors were “mainly in the brain,” he said, but cannabinoid receptors existed “throughout the body.” CB2 receptors were “mainly expressed by immune cells,” Stella pointed out, and designed to accept “endogenous cannabinoids” to regulate bodily functions.
- Stella analogized that “what THC is doing is basically hijacking this endogenous system,” which was the “same concept as with opioids.” He expected endogenous forms of cannabinoids and opioids would play a natural role in the body’s pain management.
- Behavioral Effects (audio - 5m, video)
- Stella acknowledged people had a polymodal response to cannabis which varied based on prior use, which he broke down into perceptual, cognitive, and somatic responses. He called out “chronic, high-dose” THC use as leading to “memory impairment.” Stella added that the response to CBD tended to be “very different.”
- Learning more about these effects was important for Stella’s work, especially with vulnerable populations such as youth whose brain development was “almost done” by age 20, but could continue until 25. Because the brain has many CB1 receptors for endogenous compounds to “regulate cell proliferations,” “how the neurons actually are migrating towards” areas of the brain or “establishing strong synaptic contacts,” he warned consuming cannabis could cause “dramatic rewiring” in the developing brain. This included the possibility of “psychotic episodes,” according to Stella, who wanted to see more studies “on adult cognitive function” and effects on vulnerable groups.
- Stella talked about how cannabis use disorder had been included in the Diagnostic and Statistical Manual of Mental Disorders fifth edition, DSM-5, in 2011 and included several traditional symptoms of addiction.
- Research (audio - 10m, video)
- Turning to his own research, Stella described how his lab produced “edibles for rodents” out of gelatin, measuring how much they’d ingested throughout the day. He admitted they’d seen adolescent rats eat “the THC gelatin maybe a little bit less than the normal gelatin which is something that we’re looking into,” but that after making these edibles available for a period of time they took them away to observe “what is their behavior in adulthood.” What they’d found was “big changes in the ventral tegmental area” so that adult rats presented “more risky” behavior, suggesting changes in the “reward network” that produces dopamine for the animals.
- Stella had collaborated with other researchers on techniques using “fluorescent proteins” to see CB1 receptors “in real time.” He’d seen results when scientists measured the “fluorescent signal in its mouse brain” when running indicated a response to endogenous cannabinoids. This enabled Stella’s team to better study how “THC and the endogenous cannabinoid” modulate receptors in mice.
- With THC and CBD known to reduce seizures, Stella noted Epidiolex had already received federal and state approval. He remarked that cannabinoid research had largely worked “in reverse, meaning that the field first heard about the CBD anti-seizure properties” in humans from medical cannabis patients. Stella then mentioned Charlotte Figi, a young girl who suffered “more than a hundred” epilepsy seizures a week who benefited from a high CBD cultivar called "Charlotte's Web." He added that the compound had since shown success in clinical trials and validation in “rodent models,” including those he’d been involved with.
- Confident that CBD could be “more optimized,” Stella expected this could happen through changes in the drug regimen or “chemical structure.” Instead of letting anecdotal reporting by patients drive the science, he hoped to soon “use our understanding to actually develop novel therapeutics for humans.” Areas he wanted to study in the future included autism—”a very exciting potential new therapeutic approach for this devastating disease”---sleep or anxiety issues, as well as cannabis use disorder, where “cannabidiol can actually temper the harmful effect of THC.”
- Endocannabinoid System (audio - 4m, video)
- Further study around this system would look at endogenous cannabinoid production in humans, stated Stella. He clarified the “activation of CB1 receptor on presynaptic neurons inhibit neuron transmission,” something he termed a “negative feedback loop” for THC. Another such loop occurred when “endocannabinoids on CB2 receptor block immune response,” he noted, whereas GPR55 receptors impacted by CBD “modulate neuronal activity through a very different mechanism of action.”
- Stella suggested one “therapeutic approach by having an inhibitor” like an “enzyme that eliminates endocannabinoids, or hydrolizes it” to produce “therapeutic properties that are achieved by hijacking those receptors with THC or CBD.” He named one enzyme, ABHD6, as having demonstrated potential to achieve this outcome by reducing seizures in lab animals.
- Research (audio - 6m, video)
- Among “the most exciting” areas Stella had worked on with cannabinoids was THC’s ability to kill various cancer cells. Despite a long history on the subject, he said it was more recently becoming clear the compound could have a “pronounced” benefit in glioblastoma therapy. Stella commented that the impacts “were even better with artificial cannabinoids, for example the Win[-55] compound” and this was contributing to their ideas to optimize cannabinoid therapeutics.
- He explained that between 2011 and 2017 Stella Therapeutics had worked to develop artificial molecules and “greatly improve its antitumor properties” without impacting other endocannabinoid receptors. The firm’s most recent compound, SD41, “is quite potent at killing” glioblastoma cells while carrying a “promising safety profile” for rodents, Stella indicated, calling it “one of the big efforts” published by his company.
- Stella anticipated that evidence-based models would become more prominent than “reactive” studies based on patient anecdotes, mentioning how the UW CCR was encouraging this approach “with all our partnerships.” Whether the molecule was optimized or the delivery system enhanced, utilizing the endogenous cannabinoid process or using a “medicinal chemistry effort,” he was confident that “new types of bioactivity” could be achieved.
- Legality (audio - 3m, video)
- Stella spoke to the heavy federal regulatory burden for conducting cannabis research, which had only loosened slightly in preceding years. Any cannabinoid which appeared in marijuana plants remained a schedule I controlled substance, though authorities held CBD to a different standard and Epidiolex was placed on schedule V.
- Previous cannabis samples provided through the NIH National Institute of Drug Abuse (NIDA) were "not optimal" in quality according to Stella. He hoped the system would improve so they could test cannabinoids “that are currently available on the legal market.”
- Overall, Stella found research licensing an “arduous process" and he wanted it to be more efficient by changing legal options to “enable and foster cannabinoids research and development.” As UW received federal grant dollars, it was “illegal” for them to conduct research on adult use cannabis products and, instead, “we have to go through NIH.” He advocated for both the ability to conduct research more easily and increased funding to do so.
- A Brief History (audio - 3m, video)
- Stella looked ahead to the next UW CCR webinar on May 23rd, which would be led by WSU Assistant Professor Carrie Cuttler and cover “Chronic and Acute Effects of High-Potency Cannabis on Cognition” (audio - 2m, video).
- He summed up his views on therapeutic optimization of cannabinoids by saying it was all about “how much dose is given,” its potency, regime, “what kind of device is used,” and who would use the compounds.
- Stella concluded by encouraging people to follow his work at Stella Labs and at the UW CCR, or reach out to him with any questions.
- The 2022 UW CCR Retreat was scheduled for Monday May 9th.
Information Set
-
Complete Audio - UW
[ InfoSet ]
-
Audio - UW - 00 - Complete (1h 2m 12s; May 4, 2022) [ Info ]
-
Audio - UW - 01 - Welcome - Nephi Stella (49s; May 4, 2022) [ Info ]
-
Audio - UW - 02 - Introduction - Nephi Stella (1m 59s; May 4, 2022) [ Info ]
-
Audio - UW - 03 - Introduction - UW CCR - Nephi Stella (3m 17s; May 4, 2022) [ Info ]
-
Audio - UW - 04 - Agenda - Nephi Stella (1m 47s; May 4, 2022) [ Info ]
-
Audio - UW - 05 - Bioactivity - Nephi Stella (3m 56s; May 4, 2022) [ Info ]
-
Audio - UW - 06 - History - Nephi Stella (2m 43s; May 4, 2022) [ Info ]
-
Audio - UW - 07 - THC and CBD Bioactivity - Nephi Stella (2m 14s; May 4, 2022) [ Info ]
-
Audio - UW - 08 - Scientific Approach - Nephi Stella (5m 50s; May 4, 2022) [ Info ]
-
Audio - UW - 09 - Cannabis - Nephi Stella (3m 18s; May 4, 2022) [ Info ]
-
Audio - UW - 10 - Pharmacokinetics - Nephi Stella (4m; May 4, 2022) [ Info ]
-
Audio - UW - 11 - Pharmacodynamics - Nephi Stella (3m 1s; May 4, 2022) [ Info ]
-
Audio - UW - 12 - Behavioral Effects - Nephi Stella (4m 52s; May 4, 2022) [ Info ]
-
Audio - UW - 13 - Research - Nephi Stella (9m 55s; May 4, 2022) [ Info ]
-
Audio - UW - 14 - Endocannabinoid System - Nephi Stella (3m 34s; May 4, 2022) [ Info ]
-
Audio - UW - 15 - Research - Nephi Stella (6m 18s; May 4, 2022) [ Info ]
-
Audio - UW - 16 - Legality - Nephi Stella (2m 56s; May 4, 2022) [ Info ]
-
Audio - UW - 17 - Wrapping Up - Nephi Stella (1m 42s; May 4, 2022) [ Info ]
-
-
Complete Audio - Cannabis Observer
[ InfoSet ]
-
Audio - Cannabis Observer - 00 - Complete (56m 45s; May 4, 2022) [ Info ]
-
Audio - Cannabis Observer - 01 - Welcome - Nephi Stella (1m 22s; May 4, 2022) [ Info ]
-
Audio - Cannabis Observer - 02 - Introduction - Nephi Stella (1m 37s; May 4, 2022) [ Info ]
-
Audio - Cannabis Observer - 03 - Introduction - UW CCR - Nephi Stella (4m 14s; May 4, 2022) [ Info ]
-
Audio - Cannabis Observer - 04 - Agenda - Nephi Stella (1m 52s; May 4, 2022) [ Info ]
-
Audio - Cannabis Observer - 05 - Bioactivity - Nephi Stella (3m 48s; May 4, 2022) [ Info ]
-
Audio - Cannabis Observer - 06 - History - Nephi Stella (2m 19s; May 4, 2022) [ Info ]
-
Audio - Cannabis Observer - 07 - THC and CBD Bioactivity - Nephi Stella (1m 58s; May 4, 2022) [ Info ]
-
Audio - Cannabis Observer - 08 - Scientific Approach - Nephi Stella (6m 35s; May 4, 2022) [ Info ]
-
Audio - Cannabis Observer - 09 - Cannabis - Nephi Stella (2m 11s; May 4, 2022) [ Info ]
-
Audio - Cannabis Observer - 10 - Pharmacokinetics - Nephi Stella (3m 49s; May 4, 2022) [ Info ]
-
Audio - Cannabis Observer - 11 - Pharmacodynamics - Nephi Stella (2m 15s; May 4, 2022) [ Info ]
-
Audio - Cannabis Observer - 12 - Behavioral Effects - Nephi Stella (3m 35s; May 4, 2022) [ Info ]
-
Audio - Cannabis Observer - 13 - Research - Nephi Stella (8m 22s; May 4, 2022) [ Info ]
-
Audio - Cannabis Observer - 14 - Endocannabinoid System - Nephi Stella (2m 25s; May 4, 2022) [ Info ]
-
Audio - Cannabis Observer - 15 - Research - Nephi Stella (5m 28s; May 4, 2022) [ Info ]
-
Audio - Cannabis Observer - 16 - Legality - Nephi Stella (3m; May 4, 2022) [ Info ]
-
Audio - Cannabis Observer - 17 - Wrapping Up - Nephi Stella (1m 54s; May 4, 2022) [ Info ]
-
-
UW CCR - Webinar - General Information
[ InfoSet ]
- No information available at this time