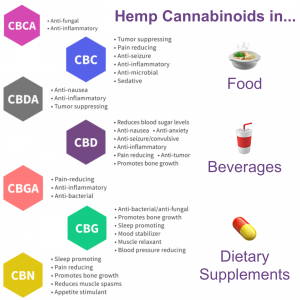
With a review of scientific literature contracted, task force members detailed remaining concerns and discussed approaches to lobbying for draft legislation on hemp cannabinoids in food.
Here are some observations from the Wednesday January 11th Washington State Hemp in Food Task Force (WA Hemp in Food Task Force) Meeting.
My top 3 takeaways:
- Staff and task force members talked through a contract to review academic literature related to human cannabinoid consumption organized by the Washington State Department of Agriculture (WSDA) at the request of task force members which would be conducted by “recently retired” Oregon State University (OSU) researcher Strat Noller.
- Several members of the task force pushed for an independent review of the science on cannabinoid limits during the group’s last meeting in December 2022.
- A revised version of the task force legislative report was shared with members and Cannabis Observer on Monday January 16th.
- Jill Wisehart, WSDA HEAL Act Coordinator, reported that the department had secured a contractor to conduct the literature review. Several participants acknowledged familiarity with the hired vendor’s work, former OSU researcher Jay Noller (who was "going by Strat [Noller] now," said Wisehart). The review was expected to take “about a month” and the contract would “remain open until May in case [of] other deliverables.” The vendor planned to meet with Concentration and Safety Work Group members the following week (audio - 1m).
- Noller participated in one task force work group meeting in October 2022.
- According to his Linkedin profile at publication time, Noller worked as the CEO of Voynich BioSciences and was Chief Visionary Officer for The van Breemen Company.
- Industrial Hemp Association of Washington (IHEMPAWA) Executive Director Bonny Jo Peterson remarked that her “original thinking was that this was being done by scientists within our state government rather than…an independent contract.” She’d since heard how such reviews “aren't traditionally done by…State staff, or legislative staff, or agency staff. I was told that that was inappropriate and it needed to be independent, but I'm not sure that everybody was aware of that” (audio - 4m).
- Wisehart didn’t agree that literature reviews were never undertaken by state officials, and instead pointed to WSDA staffing “capacity, and…we have funding remaining…from the funds from the hemp and food task force, and so it was a good way to spend some of the funds and have an external scientist do” the review.
- Jessica Tonani, Verda Bio CEO, heard "expertise and bandwidth" of department staff was the delineating factor, and since “we needed somebody that had the expertise, and we needed it done in a short period of time,” WSDA had sought an outside analysis. Wisehart concurred it was "more about the time.”
- Peterson was confident that Noller was supported by a “team of toxicologists and chemists…and microbiologists.”
- Tonani and Eric Elgar, Nextraction Vice President of Quality Operations, expressed confidence Noller was “definitely an expert in the field."
- Several members of the task force pushed for an independent review of the science on cannabinoid limits during the group’s last meeting in December 2022.
- The majority of the meeting focused on how to help the odds of potential legislation on hemp cannabinoids in food, beverages, and dietary supplements by incorporating feedback from WSDA Policy Advisor to the Director and Legislative Liaison Kelly McLain on a draft backed by some of the task force appointees.
- While the task force report to lawmakers stopped short of prescribing specific legislation to implement the group’s recommendations, in the preceding task force meetings a subset of members had extensively discussed a draft bill to set up a pilot program for hemp in food and an acceptable limit on tetrahydrocannabinol (THC) in the items’ cannabinoid content.
- Spoke Sciences Chief Regulatory Officer and Vice President of Chemistry Brad Douglass mentioned a previous idea to hold a work session or educational event on hemp in food policy for legislators and their staff, but Wisehart said that hadn’t been a task pursued “from our end” (audio - 6m).
- Tonani pointed to their counterparts on a Colorado task force who were also drafting recommendations on hemp products and described her interest in potentially making “a couple slides” in comparison to their own legislative input. She suggested the “complicated subject” would be more easily conveyed with graphics demonstrating why “we're recommending things like the ratio, why the milligram level…should be allowed.” Wisehart thought WSDA staff did “fantastic work” at graphic design and could contribute to visualizing their information.
- Peterson contemplated how they might share a bill draft with legislators, anticipating “some of them will need to see it more than once.” She named Senator Derek Stanford as a possible supporter, and emphasized he was “extremely important” to convince.
- At time of publication, Stanford was chair of the Washington State Senate Business, Financial Services, Gaming, and Trade Committee, sat on the Washington State Senate Labor and Commerce Committee (WA Senate LC) which acted as that chamber’s cannabis policy committee, and served on the Washington State Senate Agriculture, Water, Natural Resources, and Parks Committee (WA Senate AWNP) where hemp legislation was often sent.
- Stanford took questions and offered remarks during the Washington CannaBusiness Association (WACA) legislative roundtable in December 2022.
- Once she joined the meeting, McLain talked about meeting with Stanford and his staff the previous Monday because he was the “hemp cannabis guy” on WA Senate LC following his role in the 2019 legislation legalizing hemp in the state (audio - 6m).
- When she and Peterson met with Stanford, McLain found he didn’t have a “position one way or the other” on a THC limit nor whether it was set by legislators or regulators at WSDA, “although he does believe in the kind of nimble abilities of an agency to be able to do some of that work on their own, rather than having it solidified in statute.”
- While not likely to sponsor the measure, McLain relayed that Stanford had assisted in identifying other possible sponsors. She further noted that the version of the bill she was aware of would “go through…the agriculture committee unless it was grabbed on purpose by…the regulated substances committees.” McLain stated that she’d advise legislators the draft bill “doesn't belong in with the high-THC type bills” heard by those committees. She announced that committee staff were editing the draft language into something that could be put into bill form by the Washington State Office of the Code Reviser (WA OCR). Moreover, she expected the resulting wording would "more closely align with the existing food safety regulatory system so that the costs drop a little bit for the agency to do the work.” McLain expected the formatted document would be available in the next couple days and be shared with the full task force.
- McLain added that she was “fully expecting a battle over where LCB [Washington State Liquor and Cannabis Board] is at on numbers in their [cannabinoid regulation] bill and where this bill ends up with the THC issue.” McLain commented that she “prepped” WA House RSG Co-Chair Shelley Kloba on both bills, and would be following up to convey “how these two bills are playing out, and why they're different.”
- A significant unknown about the proposed regulation, she said, was that when regulating a “marketplace,” the State relied upon enforcement by the “local public health jurisdictions…and their costs are usually exceptionally high.” McLain planned to talk to a lobbyist with the Washington State Association of Local Public Health Officials (WSALPHO) about the measure the following day.
- Tonani talked about the educational effort that some task force members were engaged with, telling McLain that when lobbying in 2022, she’d heard that there wasn’t a budget to “prevent some of the…potentially unsafe products in the market.” Tonani wondered if the legislation could include money to target retail removal of unregulated cannabinoid products sold outside the adult-use cannabis sector (audio - 4m).
- Peterson agreed that knowing whether there were “two separate buckets” for regulating hemp in food items versus removing unregulated items was important for effective lawmaker education and lobbying.
- McLain argued it was a matter of “efficiency and economy of motion” with state and local regulators working together to oversee hemp products. However, she emphasized that local enforcement tended to be complaint based, and that health officials weren’t expected to be “out there looking at the labels of every single product that's available.” McLain then noted how the “majority of the safety factor stuff is going to happen on the manufacturing side.”
- Having discussed a $2.4 million estimated fiscal note for the draft legislation, Peterson sought clarification over whether local enforcement costs were included. McLain replied that they were “likely two separate buckets" but she would get an idea of the cost from the WSALPHO once their representatives “see the bill language”(audio - 1m).
- Dave Wyckoff, CEO of Wyckoff Farms, brought up the THC limits in the draft bill, wondering if McLain could offer an official WSDA position or describe how WSDA “sees itself relating to LCB on the subject, where LCB has set a different standard” in their legislation (audio - 4m).
- McLain was careful to say the department was “not taking a position on the THC” because the compound wasn’t a “regulatory metric that WSDA would be enforcing” unless directed to by statute, and no one from the department would be testifying to legislators “what the right THC level is.” Instead, she had asked staff to consider, based on “how the bill is written, can we implement it?”
- The department didn’t plan to take a position on the appropriateness of THC limits in either the hemp in food proposal or the WSLCB legislation, she said, and if the hemp bill was introduced and given a hearing, “what you would get was me testifying ‘other.’” While not opposing the bill, McLain would take no position on appropriateness of THC levels, stating “all the comments you've gotten back from us and all of the edits that I'll make are to streamline it such that we could do a fiscal note on it; we could implement the bill if it was funded.” Her comments about the topic would be “as close to support as I can get on non-agency request, or Governor's approved legislation.”
- Wyckoff said the bill draft “speaks in terms of joint agency authority, that is under the WSDA, but include[d]” Washington State Department of Health (DOH) officials as well. McLain said she was waiting to hear back from a colleague at DOH who had received a copy of the draft, but their first reaction was that “local health needs to be at the table” as those agencies would execute most of the responsibilities the bill designated to DOH. She added that Policy Director “Joe [Laxson] from DOH” Division of Environmental Public Health would join her at the WSALPHO meeting the following day, but her impression was “they're going to defer to us on most of the structure of the program unless they see a fatal flaw that they can't Implement in their existing programs” (audio - 2m).
- Laxson last attended a task force meeting on behalf of DOH in November 2022.
- Wyckoff brought up comments previously shared on the draft bill by WSDA food safety staff, indicating he didn’t understand their “comments related to section 4, subsection 1 as it relates to seemingly approving the production for ingredients sales outside of the state, but not in the state.” Peterson agreed the section included language she considered “confusing” conflating certification and licensing hemp processors. McLain concurred the issue was an “easy thing that we can fix when I get the bill language back,” involving hemp “for sale in states that allow hemp extract as a food ingredient right now” (audio - 3m).
- The subject of “whether third party…audits of GMPs [good manufacturing practices] will be acceptable” was another area Wyckoff wanted to understand along with whether companies would be licensed or certified to manufacture hemp in food (audio - 7m).
- McLain reported that hemp would be an “approved food ingredient, and then we would treat it like any other food processor license. And so…that is the cleanest way for it to operate within our existing food safety structure.” She mentioned some lingering concerns about safety inspectors being “dual certified to inspect under the federal” guidelines for food inspection and whether existing staff could look at facilities when federal authorities had been “very clear that this is not a food ingredient in their eyes.” This had already increased the cost of hemp in food legislation, she stated, “because it is likely that we will have to have specialized…inspectors that just do this work.”
- McLain and Tonani established that their ideal scenario was one in which WSDA staff that inspected cannabis edibles facilities would be supported to also cover hemp in food businesses.
- McLain elaborated that the department didn’t have experience contracting outside firms to do those kinds of inspections, in part since federal regulators “will not allow those third-party certifications, even if they meet the exact same criteria.” Wyckoff suggested that third party inspectors be allowed on a temporary basis “just to get the program up and running without any significant delay.” McLain agreed it was possible those kinds of inspections could be done during the pilot program and Wyckoff suggested a few “readily accepted” auditing firms.
- David Gang, Washington State University Center for Cannabis Policy, Research, and Outreach (WSU CCPRO) Director, followed up on the “selection process” for third-party inspectors (audio - 3m).
- McLain said there were multiple approaches to this, but she preferred modeling a program after the “livestock ID program…where we couldn't meet all of the livestock and branding needs in the state. And so we actually offer a training and we certify private veterinarians and others. They come in and…get a certificate from us” in order to “act as a livestock ID agent for the State of Washington.” Inspection criteria could be outlined in law or rule, she noted, alongside “qualifications for the third party auditor. And then any third party audit that is submitted has to show that they meet those criteria.” The latter system was probably the “easiest,” McLain acknowledged. Wyckoff promised to send her “audit standards” used by other firms, and McLain welcomed input ahead of WSDA staff setting fee structures for inspections.
- Peterson suggested tying use of third party auditors to the pilot program included in the draft bill. McLain felt WSDA representatives were amenable to having that policy last longer, but it would depend on “identifying what the confines and structure would be for the third party” (audio - 3m).
- Tonani called out “food safety audits” which had been used in the cannabis industry, asking that department staff “think of those companies” while they’re preparing any third party inspection guidance. McLain welcomed information about those audits so WSDA officials could ensure “it meets…the level of rigor that we would expect from a state program.”
- The inclusion of an emergency clause—allowing a bill to take faster effect—was Wyckoff’s last inquiry, as he had the impression that this would allow for expedited rulemaking by WSDA (audio - 2m).
- “We are not able to use expedited, or emergency rulemaking,” McLain commented, absent specific allowance in legislation or circumstances involving “potential loss of human health or potential egregious environmental harm.” She continued, telling the group “normally, if you file for an expedited rule, if one person for any reason challenges that, it jumps you all the way back into the normal rulemaking process. So, what we did with hemp when the program was created, we did expedited rulemaking authority explicit[ly],” meaning, “no one can bump us out of expedited rule making and we're able to move more quickly.”
- The drawback, according to McLain, was “ideally, we don't use expedited rulemaking all the time” but instead limit use to situations like “establishing a program, or [if] you need to make a quick shift. But you don't use it all the time because it really does shortchange the public on public engagement during rulemaking.”
- Expecting she would revise a draft of the potential bill by the weekend, McLain concluded, “even though the agency can't support this because of the fiscal note, we can support the policy behind it, and I'm happy to help work on that” (audio - 1m).
- When Gang inquired about the staffing expectations for a potential bill, McLain responded that she couldn’t be sure until she had “clean bill language,” but doubted it could be administered with less than two full time staffers. Another factor she noted was the rate at which other companies wanted to get licensed under the program, adding it was possible to have “exponential growth potential” lead to significant licensing delays if not planned for by regulators (audio - 6m).
- Whatever the initial costs to stand up the program, McLain articulated the goal of WSDA was creation of a program that was “fully cost recoverable and the State would[n’t have to] be spending any money on it.” Peterson disagreed, preferring to avoid “that cycle of self-supporting.” But McLain said that as “sixty percent of the agency actions are self-supporting fee-for-service work specifically because there is no public benefit nexus to the work that's happening, it is to the benefit of an industry that is money making, and therefore that industry has to pay for the service.” She expected “the state covers the cost until the program is fully stood up and then the program covers its costs going forward,” if for no other reason than “we couldn't even do a full idea on what the fees should be for the program, until we know what the program looks like size wise. So, it would likely be two to three years that the state would fund the cost of setting up the program” in order to make a hemp in food program sustainable.
- McLain added that licensed cannabis businesses “pay for the services they receive from LCB in the form of a very large tax structure,” and “until we actually have the bill in front of us” she couldn’t speak to “what does it really take to stand up the program, and sustain the program, annually and biennially.”
- After Tonani posited that there was a public safety benefit in taking enforcement action on unregulated hemp products as the sector got started, McLain agreed, optimistic she could “make a really good argument for the first couple years that we need to do this,” but “the program will have to sustain itself at some point.”
- Asked where enforcement funding would end up going, McLain reiterated that most would go to local health agencies who would then rank it “in priority with the other things that they don't want in…those spaces” such as “access to vape pens and all sorts of other things. They would do it as part of their normal local public health duties in the retail space” (audio - 1m).
- Wyckoff stated that hemp oversight being self-sustaining made sense to him. Then Peterson and McLain discussed when a business would be expected to obtain both a hemp producer and processor license, and how those costs might support a hemp in food program (audio - 4m).
- As the meeting wrapped up, several members looked at the timeline for action on any potential bill and decided not to attempt a formal vote on a THC limit for the legislation.
- Asked about the legislative session cutoff calendar, McLain replied that bills had until February 17th to be passed by an initial policy committee. But given the expected costs of implementation, she articulated a goal to “move it quicker out of [the policy committee] so you can get it to the fiscal committee and get it heard there.” Tonani suggested another task force call be scheduled once a final version of the proposed bill was distributed to task force members (audio - 2m).
- Peterson mentioned a vote on the draft bill’s THC limit for hemp products, but Tonani didn’t see that number as impacting how they wanted to proceed on the legislation (audio - 1m).
- At time of publication, the task force had not announced their next meeting and no hemp-related legislation had been introduced in the legislature.
Information Set
-
Complete Audio - Cannabis Observer
[ InfoSet ]
-
Audio - Cannabis Observer - 00 - Complete (1h 1m 29s; Jan 11, 2023) [ Info ]
-
Audio - Cannabis Observer - 01 - Literature Review - Jill Wisehart (1m 13s; Jan 11, 2023) [ Info ]
-
Audio - Cannabis Observer - 02 - Literature Review - Question - Background - Bonny Jo Peterson (3m 41s; Jan 11, 2023) [ Info ]
-
Audio - Cannabis Observer - 03 - Draft Legislation - Question - Timing - Jessica Tonani (26s; Jan 11, 2023) [ Info ]
-
Audio - Cannabis Observer - 04 - Legislative Report - Question - Status - Bonny Jo Peterson (53s; Jan 11, 2023) [ Info ]
-
Audio - Cannabis Observer - 05 - Draft Legislation - Question - Educating Officials - Brad Douglass (5m 49s; Jan 11, 2023) [ Info ]
-
Audio - Cannabis Observer - 06 - Draft Legislation - Kelly McLain (5m 43s; Jan 11, 2023) [ Info ]
-
Audio - Cannabis Observer - 07 - Draft Legislation - Question - Enforcement - Jessica Tonani (4m 16s; Jan 11, 2023) [ Info ]
-
Audio - Cannabis Observer - 09 - Draft Legislation - Question - WSDA Position on THC Limits - Dave Wyckoff (3m 52s; Jan 11, 2023) [ Info ]
-
Audio - Cannabis Observer - 10 - Draft Legislation - Question - DOH Position - Dave Wyckoff (1m 35s; Jan 11, 2023) [ Info ]
-
Audio - Cannabis Observer - 12 - Draft Legislation - Question - Third Party Audits - Dave Wyckoff (6m 37s; Jan 11, 2023) [ Info ]
-
Audio - Cannabis Observer - 13 - Draft Legislation - Question - Third Party Auditors - David Gang (3m 27s; Jan 11, 2023) [ Info ]
-
Audio - Cannabis Observer - 14 - Draft Legislation - Question - Emergency Clause - Dave Wyckoff (2m 23s; Jan 11, 2023) [ Info ]
-
Audio - Cannabis Observer - 15 - Draft Legislation - Kelly McLain (46s; Jan 11, 2023) [ Info ]
-
Audio - Cannabis Observer - 17 - Draft Legislation - Question - WSDA Staffing - David Gang (6m 2s; Jan 11, 2023) [ Info ]
-
Audio - Cannabis Observer - 19 - Draft Legislation - Comment - Self-Supporting Program - Dave Wyckoff (4m 7s; Jan 11, 2023) [ Info ]
-
Audio - Cannabis Observer - 20 - Draft Legislation - Question - Cutoff Deadlines - Bonny Jo Peterson (1m 48s; Jan 11, 2023) [ Info ]
-
Audio - Cannabis Observer - 21 - Wrapping Up - Jill Wisehart (18s; Jan 11, 2023) [ Info ]
-
Audio - Cannabis Observer - 22 - Draft Legislation - Question - THC Limits - Bonny Jo Peterson (53s; Jan 11, 2023) [ Info ]
-
Audio - Cannabis Observer - 23 - Wrapping Up - Jill Wisehart (15s; Jan 11, 2023) [ Info ]
-
-
WA Hemp in Food Task Force - Meeting - General Information
[ InfoSet ]
-
Announcement - v1 (May 16, 2022) [ Info ]
-
Announcement - v2 (May 18, 2022) [ Info ]
-
Document Repository - WSDA (Sep 6, 2022) [ Info ]
-
Legislative Report - v3 (Nov 30, 2022) [ Info ]
-
Legislative Report - v4 (Jan 12, 2023) [ Info ]
-