Director Rick Garza discussed agency efforts to understand delta-8-THC and develop policy following increased momentum towards restriction by other states amid federal uncertainty.
Here are some observations from the Tuesday March 16th Washington State Liquor and Cannabis Board (WSLCB) Board Caucus.
My top 3 takeaways:
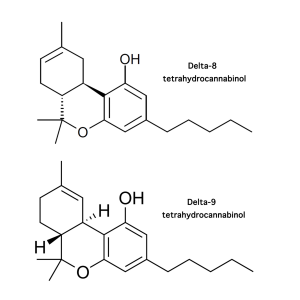
- Since federal legalization of hemp, questions about inclusion of the compound delta-8-tetrahydrocannabinol (delta-8-THC) in legal cannabis products had troubled federal and state regulators.
- Delta-8-THC, a delta-9-THC isomer with minor differences in chemical bonds, grew increasingly prominent following passage of federal legislation which legalized hemp production in 2018.
- In 2018, the World Health Organization (WHO) Expert Committee on Drug Dependence released a review on the isomers of THC which covered delta-8-THC identification, chemistry, and the “Ease of Convertibility Into Controlled Substances.”
- In August 2020, the U.S. Drug Enforcement Administration (DEA) published a request for public comment on the agency’s interim final rule on implementation of the 2018 Agricultural Improvement Act (the Farm Bill) which “merely conforms DEA’s regulations to the statutory amendments to the [federal Controlled Substances Act] CSA that have already taken effect, and it does not add additional requirements to the regulations.” The public comment period ended in October 2020 and the rule was expected to become final in April.
- The rule “does not impact the control status of synthetically derived tetrahydrocannabinols (for Controlled Substance Code Number 7370) because the statutory definition of ‘hemp’ is limited to materials that are derived from the plant Cannabis sativa L.” Though delta-8-THC was not specifically addressed by the rule, it states that “the concentration of [delta-]9-THC is not a determining factor in whether the material is a controlled substance. All synthetically derived tetrahydrocannabinols remain schedule I controlled substances.”
- This language led to speculation about the legislative intent which distinguished “synthetically derived” THCs---which case law indicates includes Spice and K2, as well as dronabinol which is not derived from the cannabis plant and the DEA labels “synthetic”---in contrast to organically derived THCs which are spelled out in the Farm Bill definition of hemp as “all derivatives, extracts, cannabinoids, isomers.”
- Distinctions between plant-based extraction, chemical synthesis, and biosynthesis are explored in this prospectus bullish on the prospects for cannabinoid production into active pharmaceutical ingredients (API). Some methods for deriving delta-8-THC from cannabidiol (CBD) rely on chemical solvents. At publication time, those methods were not regulated and end product testing was not required for hemp products.
- In November 2020, Policy and Rules Manager Kathy Hoffman incidentally mentioned WSLCB staff were paying attention to delta-8-THC.
- In late 2019, the WSLCB Cannabis Potency Tax Work Group made note of delta-8-THC in its final report for the Legislature, indicating that “while delta-9 THC is the most prevalent cannabinoid and intoxicant in the cannabis plant, there are other cannabinoids, for example delta-8 THC, that potentially contribute to the effects or potency of the cannabis plant.”
- In June 2020, WSLCB Chemist Nicholas Poolman described his understanding of how some licensees were “bringing in CBD isolate from hemp sources to their facilities, chemically synthesizing the CBD into Δ9-THC and Δ8-THC, and then moving these products into the market.”
- During the fall of 2020, WSLCB representatives mentioned delta-8-THC in draft conceptual rules for the quality control (QC) testing and product requirements rulemaking project, which stated, “Any psychoactive cannabis derivative intentionally added to the formula of a product must be tested for potency, including but not limited to delta-8.” The proposed QC rules were modified to state, “Any psychoactive cannabinoids intentionally added to the formula of a product must be tested for potency.” At publication time, further revisions to the proposed QC rules were being made by WSLCB staff.
- On February 2nd, Hoffman provided an update on her work drafting a formal policy statement on delta-8-THC which articulated agency concerns.
- The statement indicates obtaining delta-8-THC from CBD “may generate additional chemicals that are not naturally occurring in cannabis” with unclear health implications.
- The draft concluded, “any product that contains any amount of any synthetically derived delta-8-THC or delta-9 THC” was prohibited and “until the agency has both authority and verifiable information on which to base its decision making regarding delta-8-THC or any other isomer of THC, regardless of the form of THC, the total THC amount cannot exceed that allowed per serving as specified in WAC 314-55-095.”
- On February 3rd, a licensee called for regulating “the unintended consequences of cannabinoids coming into the system” from “huge amounts of hemp biomass.”
- On February 16th, Hoffman reviewed the draft policy statement with the Board, saying it had been distributed to particular stakeholders who had until February 22nd to provide comment.
- Delta-8-THC, a delta-9-THC isomer with minor differences in chemical bonds, grew increasingly prominent following passage of federal legislation which legalized hemp production in 2018.
- Director Rick Garza described the agency’s position and activities around delta-8-THC in Washington state as well as engagement with Oregon regulators and the federal government via the Cannabis Regulators Association (CANNRA, audio - 5m).
- Garza first pointed to the Oregon Liquor Control Commission (OLCC) commencement of an “Effort to Limit Unchecked Use of Delta-8-THC, Other Artificially-Derived Cannabinoids” following a meeting attended by WSLCB staff. OLCC and other Oregon agencies such as the “Department of Ag[riculture], Department of Health, the Board of Pharmacy” led a presentation ”with respect to this new cannabinoid” which Garza commented had already shown up in Oregon and Washington markets.
- “We’re kind of late in the legislative process to introduce a bill,” Garza said, whereas he understood that Oregon lawmakers would introduce a bill on the topic “in the next week or two to figure out how they’re going to get a hold of this delta-8-THC product that they’re finding.”
- On February 10th, WSLCB leadership acknowledged they had no “role in overseeing or regulating hemp" but intended to lobby lawmakers on SB 5372 ("Concerning a hemp processor registration process") to amend the bill so WSLCB staff could “test samples represented as hemp obtained from a location licensed for marijuana production or processing for the sole purpose of validating THC content” - which would presumably include hemp-derived products containing delta-8-THC. On March 9th, senators voted to adopt the amendment just before passing SB 5372 unanimously. On March 19th, Director of Legislative Relations Chris Thompson explained the agency’s requested amendment during the bill’s House policy committee public hearing (audio - 3m, video).
- Garza explained that delta-9-THC was the sole THC compound identified in the two states’ regulatory systems “when we put them together.” He admitted he didn’t “understand the chemistry of it” but felt delta-8-THC was “not a natural derived product, it’s being chemically altered" and was a “by-product.” He added there was concern from regulators because “we don’t know if there’s THC in that product. Typically, there will be small amounts of it when it’s naturally derived from the cannabis plant but there’s a lot we don’t know about this product.”
- Garza reported that the Cannabis Regulators Association (CANNRA), in which he served as First Vice President, subsequently convened to discuss delta-8-THC with assistance from University of Washington (UW) Department of Health Services Clinical Instructor Gillian Schauer. He said Schauer had been contracted by WSLCB “to help us do some research on this issue and others.” She “brought the states together” through CANNRA in order to hear “what are they doing in response to this issue around delta-8.”
- In November 2020, Hoffman stated Schauer represented prevention and public health views to the newly formed CANNRA and had previously briefed regulators on those subjects.
- According to a WSLCB spreadsheet of active contracts, Schauer’s contract began on February 15th and would end June 30th, 2021. She had previously consulted for the Centers for Disease Control and Prevention (CDC) and the National Institute on Drug Abuse (NIDA).
- Garza concluded by noting the work of agency staff along with the Washington State Office of the Attorney General (WA OAG) to “determine the authority that we have by rule to bring this particular cannabinoid under regulation as we do [for] delta-9.” He expected to have a proposal to show to the Board “very soon” because scrutiny of delta-8-THC was now “happening all over the country” especially since it was “present outside of the regulated cannabis marketplace in other products.”
- Board Member Russ Hauge spoke up to say he’d had “a little involvement” helping agency staff draft the delta-8-THC policy and offered to be the “board liaison on this issue.” He added that the topic of “synthetics is going to be with us for a while” (audio - 1m).
- In his first board caucus as Chair, David Postman sought to understand the federal position on delta-8-THC.
- The newly appointed Postman asked whether staff perceived “rumblings of federal action on this” as it would impact “states with legalized [cannabis] markets and those without” (audio - 2m).
- Garza told him that following the last CANNRA meeting, members resolved to “get a meeting together with the [Food and Drug Administration] FDA.”
- He continued, saying some states’ Boards of Pharmacy had been empowered to “determine that this, this new cannabinoid can be restricted and scheduled and then cannot be legal in those states. That’s a pretty drastic step” but some “states are employing that.”
- Another approach was regulation of “all cannabinoids...and I think that’s what we’re going to see in Oregon,” Garza said “because there’s lots of different cannabinoids that can be created” but delta-8-THC wasn’t “a naturally occurring cannabinoid the way that it’s being created.”
- The newly appointed Postman asked whether staff perceived “rumblings of federal action on this” as it would impact “states with legalized [cannabis] markets and those without” (audio - 2m).