Given two proposed substitutes on a bill to regulate cannabinoids, almost the entire committee voted for a more limited version to get some cannabinoid products off the shelves and study the issues.
Here are some observations from the Monday March 7th Washington State Senate Ways and Means Committee (WA Senate WM) Committee Meeting.
My top 4 takeaways:
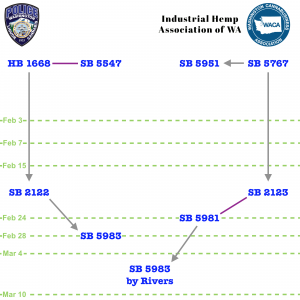
- Two paths were offered for the revision of SB 5983, "Concerning untested and unregulated cannabinoid products," incorporating input from public testimony days earlier to varying degrees.
- When the bill received a public hearing on March 5th showing a clear divide on its potential effectiveness and stakeholder impacts, the sponsor, Senator Karen Keiser, promised amended language was forthcoming.
- See Gregory Foster’s comments in the Week Ahead, which arrived on a four-year anniversary of HB 2334, the bill which authorized the import of hemp cannabidiol (CBD) into Washington’s closed legal cannabis market.
- Washington State Senate Labor, Commerce, and Tribal Affairs Committee (WA Senate LCTA) Counsel Matt Shepard-Koningsor briefed on two proposed substitutes, one from Keiser, and the other by Senator Ann Rivers (audio - 5m, video).
- Amendment A (S-5402.2) by Rivers would replace the bill language with a slightly modified version of SB 5981, which Rivers co-sponsored as companion legislation to HB 2123, and had the following effects:
- “Adds a definition of ‘Plant Cannabis’ in the Uniform Controlled Substances Act (UCSA), which means all plants of the genus Cannabis, including cannabis and hemp;
- Removes proposed qualifying language in the definition of ‘tetrahydrocannabinol’ or ‘THC’ that stated THC included concentrated resins or cannabinoids and their products, produced from the plant Cannabis, whether or not the cannabinoids were derived from a cannabis plant containing a THC concentration greater than 0.3 percent on a dry weight basis;”
- The revised definition reads: “(vv) ‘Tetrahydrocannabinol’ or ‘THC’ includes all tetrahydrocannabinols that are artificially, synthetically, or naturally derived including, but not limited to, delta-8 tetrahydrocannabinol, delta-9 tetrahydrocannabinol, delta-10 tetrahydrocannabinol, THCv tetrahydrocannabivarin, THCP tetrahydrocannabiphorol, THC-O-Acetate, and the optical isomers of THC cannabinoids.”
- “Amends the definition of ‘THC concentration’ in the UCSA to include all tetrahydrocannabinols instead of only delta-9 THC;
- Provides that products containing or consisting of cannabinoids produced and processed for human consumption, whether marketed as such or not, exceeding a THC concentration of 0.3 percent, may only be sold by a licensed cannabis producer, processor, or retailer unless authorized as a drug by the federal Food and Drug Administration. Unadulterated and unprocessed hemp flower is exempted;”
- At publication time, the Washington State Pharmacy Quality Assurance Commission (WA Pharmacy Commission) was engaged in rulemaking to deschedule Epidiolex, a CBD-based pharmaceutical drug.
- “Provides that products containing a THC concentration of 0.3 percent or less sold by any person other than a state cannabis licensee must contain a 20:1 ratio of cannabidiol (CBD) or other non-THC cannabinoids to THC, and must not exceed two milligrams of THC per serving. Unadulterated and unprocessed hemp flower is exempted;”
- Shepard-Koningsor thought this meant there needed to “be twenty times as much CBD…as THC in the product.”
- This, and the preceding effect, incorporated public testimony from hemp interests during the hearing.
- “Requires the Washington State University Center for Cannabis Policy, Research, and Outreach, contingent on funding, to convene a scientific panel (Panel) that will review available research, data, and regulations of other jurisdictions relative to defining the terms ‘impairing,’ ‘artificial cannabinoids,’ and ‘synthetically derived cannabinoids,’ in addition to making recommendations on safe manufacturing, extracting, and synthesizing of cannabinoids. The Panel must report to the Legislature by December 1, 2022;
- Directs the Department of Health, contingent on funding, to establish a grant program to assist local health departments enforce unauthorized sales of cannabinoids;
- Contains a severability and an emergency clause relative to certain sections in the bill;
- Changes the term ‘marijuana’ to ‘cannabis’ in the new bill language; and
- Modifies the title and intent section language.”
- Amendment B (S-5410.2) by Keiser revised the legislative text with the following changes:
- “Replaces ‘may be impairing’ with ‘generally considered or marketed as impairing’ in relation to a cannabinoid throughout the bill; Removes the definition of ‘impairing’ in relation to a cannabinoid;”
- Instead a definition would be “defined, in rule, by the LCB,” Shepard-Koningsor told the committee.
- “Adds ‘hexahydrocannabinol’ to the listed examples contained in the definition of ‘cannabinoid’;”
- Abbreviated HHC, Washington State Liquor and Cannabis Board (WSLCB) Director of Legislative Relations Chris Thompson drew attention to the compound on February 1st when discussing classifications of cannabinoids on another bill pertaining to hemp, SB 5951. He claimed HHC had been “reported to be more intoxicating than delta-8 and maybe a little less than delta-9” and stated it was “apparently, quite demonstrably intoxicating.”
- In conversation with chemists, Cannabis Observer learned some believe HHC may not be a naturally occurring compound in cannabis plants, and would therefore fall under the definition of an artificial cannabinoid first and foremost.
- Allows the sale of products: (1) Containing cannabinoids that are generally considered or marketed as impairing; (2) containing greater than 0.3% tetrahydrocannabinol (THC); or (3) containing 0.5 or more milligrams (mg) per serving or two or more mg. total in the packaged product, if the product contains a cannabidiol (CBD) to THC ratio of 20:1 (i.e., there must be 20 times as much CBD than THC in the product);
- “The difference between this one and the other proposed sub is that this limits it to CBD, and it does not allow the other non-THC cannabinoid in the ratio,” Shepard-Koningsor reported.
- Requires the Washington State University Center for Cannabis Policy, Research, and Outreach and LCB to convene a scientific panel (Panel) consisting of members with scientific expertise in a number of concentrations and representatives from the Department of Health, Department of Agriculture, and LCB. The Panel must review information in other jurisdictions relative to cannabinoids and report to the Legislature by December 1, 2023;”
- Compared with the panel in Rivers’ proposal, Keiser’s CCPRO panel would involve a broader collection of state agencies to provide a review of other states’ policies, and wouldn’t be required to make recommendations “on safe manufacturing, extracting, and synthesizing of cannabinoids.” Keiser’s language would also give the panel more than a year to complete their work, whereas Rivers’ panel would have to submit both their review of other jurisdictions and recommendations by December 2022.
- “Establishes an LCB advisory committee to review issues and topics of interest regarding regulating cannabinoids, which must be composed of, at minimum, cannabis licensees, industry associations, public health professionals, and representatives from other relevant state agencies;
- Includes businesses classified as ‘gasoline stations with convenience stores’ as described in the North American Industry Classification System in the list of businesses exempt from the temporary surcharge;
- Changes the term ‘marijuana’ to ‘cannabis’ in the new bill language; and
- Modifies the title and intent language.”
- “Replaces ‘may be impairing’ with ‘generally considered or marketed as impairing’ in relation to a cannabinoid throughout the bill; Removes the definition of ‘impairing’ in relation to a cannabinoid;”
- WA Senate WM Senior Fiscal Analyst Amanda Cecil discussed the fiscal note released earlier that day, explaining it covered costs anticipated by four state agencies. Cecil informed the committee that the majority of the identified costs were for the WSLCB and Washington State Patrol (WSP, audio - 1m, video).
- Washington State Office of Attorney General - Estimated cash receipts and operating expenditures “are assumed to equal the Legal Services Revolving Account (LSRA) cost estimates. These will be billed through the revolving account to the client agency,” the WSLCB.
- Fiscal Year (FY) 2021-23 - $113,000
- FY 2023-25 - $122,000
- FY 2025-27 - $30,000
- Washington State Department of Revenue - Estimated operating expenditures were driven by the department’s labor costs helping ensure the temporary licensing fee surcharge would be collected.
- FY 2021-23 - $77,900
- WSLCB - Operating expenditures were expected for implementation, ongoing rulemaking, enforcement, litigation, and information technology costs.
- FY 2021-23 -$1,654,724
- FY 2023-25 - $1,529,604
- FY 2025-27 - $521,740
- WSP - Estimated operating expenditures were primarily associated with the WSP Crime Laboratory Division (CLD) which “need[s] to provide evidence testing that meets the new definitions of the proposed legislation.” Costs would include additional equipment, staffing, and contracting costs.
- FY 2021-23 - $1,655,000
- According to the fiscal note, the WSP CLD “will need to acquire [three] new Liquid Chromatography-Mass Spectrometry (LC/MS) instruments capable of conducting the more detailed analysis required. It will also be necessary to develop and validate new testing protocols for the new instruments and then train staff on these protocols.”
- FY 2023-25 - $254,000
- FY 2025-27 - $254,000
- FY 2021-23 - $1,655,000
- Washington State Office of Attorney General - Estimated cash receipts and operating expenditures “are assumed to equal the Legal Services Revolving Account (LSRA) cost estimates. These will be billed through the revolving account to the client agency,” the WSLCB.
- Keiser asked about the difference in WSLCB rulemaking power granted between the proposed substitutes, pointing out that her proposal allowed the agency to do rulemaking for “all cannabinoid products” while alleging under Rivers’ proposal they “may not engage in rulemaking for all cannabinoid products.” Shepard-Koningsor acknowledged that “explicit authority” for new rulemaking was only in her version along with new definitions, though he made clear the agency had “general rulemaking authority in a separate section of the statute” and was not permitted to regulate hemp production (audio - 2m, video).
- Senator Bob Hasegawa asked staff whether there was a document with a side-by-side comparison of the substitutes, and whether either had a definition for ‘impairing.’ Shepard-Koningisor replied that there was a side-by-side document being drafted, and that while the original text of SB 5983 had a definition for impairing, Keiser’s proposed substitute removed it and Rivers’ proposal hadn’t included one (audio - 1m, video).
- Senator Mark Mullett asked if Rivers’ proposed substitute would get unregulated cannabinoid products out of convenience stores. Shepard-Koningsor told him items “over the 0.3% THC limit” would be prohibited “from being sold outside of the [legal cannabis] market…but it does allow other products under the 0.3% THC threshold to be sold in those stores if they meet” the 20:1 ratio of other cannabinoids to THCs. Mullet followed up to see if the products used as “props” during the public hearing would be banned under the bill. Shepard-Koningsor answered that the example product he’d seen, a pack of delta-8-THC gummies totaling “500 milligrams…would be prohibited,” but products with lower amounts could be permitted under Rivers’ language (audio - 2m, video).
- Hasegawa understood trying to “get a grip on all the impairing” compounds, but remained concerned about potential impacts to the “non-impairing” and CBD portions of the hemp market. Would WSLCB rules “possibly negatively impact those other areas,” he wondered. Shepard-Koningsor said the agency would have power related to “products that are generally considered or marketed as impairing,” granting WSLCB leaders “discretion” in their rule development. But they didn’t have authority “to adopt rules on hemp,” which was regulated by the Washington State Department of Agriculture (WSDA, audio - 2m, video).
- When the bill received a public hearing on March 5th showing a clear divide on its potential effectiveness and stakeholder impacts, the sponsor, Senator Karen Keiser, promised amended language was forthcoming.
- The authors of the proposed substitutes introduced their amendments in turn, giving members the chance to ask questions to evaluate which revision best responded to the pressing issues and concerns they had heard.
- Amendment A (S-5402.2.) - Rivers said the genesis of her proposed substitute was SB 5981, which she’d co-sponsored, noting it hadn’t been granted a hearing in the WA Senate LCTA. She observed that during public testimony on the bill, most everyone had voiced concern about products being sold outside of the licensed cannabis market and wanted decisions “made in science.” Rivers indicated she had found stakeholder engagement lacking “after [the WSLCB request bill on the topic, HB 1668] died” on the House floor. Not wanting to continue the “controversy” of giving “unfettered rulemaking” power to WSLCB and other things requested in their bill, she decided to take “out all of the disagreeable stuff” (audio - 2m, video).
- Senator Steve Conway wanted to know why DOH would be granted enforcement authority in this area. Rivers answered that hemp in food was under DOH jurisdiction, giving them a role in stopping unregulated cannabinoid products. She added that she’d heard from “several in the retail association that they had received no outreach” from WSLCB when drafting regulations. Rivers believed DOH officials might “have lines of communication that would better suit disseminating the information” to convenience and grocery stores, as they had done so “when we legalized the distribution of hemp” (audio - 2m, video).
- At publication time, WSDA leaders had set up rules around hemp production, completed rulemaking for Hemp Processor Registration in December, and undertaken a rulemaking project for Hemp Extract Certification. They had not undertaken rulemaking on the use of CBD in food, but other hemp products could be included like “hulled hemp seeds, hemp seed protein power[sic] and hemp seed oil, provided they comply with all other requirements. FDA has determined that these components are Generally Recognized as Safe (GRAS) based on federal requirements.”
- Although DOH had not established rules for cannabinoid use in food, they had a significant role in food safety.
- Find out more from the WSLCB Hemp/CBD frequently asked questions.
- Senator John Braun asked whether a change in WSLCB authority was necessary as there seemed to be disagreement on that point, in contrast to broad agreement on the need to ban “the unregulated product in anything other than a licensed facility.” Shepard-Koningsor stated that WSLCB had analyzed the substitutes and suggested Rivers’ version wouldn’t give them “the authority that they need to prohibit those products” in part due to the lack of a definition of ‘cannabinoid’ (audio - 2m, video).
- Senator Steve Conway wanted to know why DOH would be granted enforcement authority in this area. Rivers answered that hemp in food was under DOH jurisdiction, giving them a role in stopping unregulated cannabinoid products. She added that she’d heard from “several in the retail association that they had received no outreach” from WSLCB when drafting regulations. Rivers believed DOH officials might “have lines of communication that would better suit disseminating the information” to convenience and grocery stores, as they had done so “when we legalized the distribution of hemp” (audio - 2m, video).
- Amendment B (S-5410.2) - Keiser remarked that the existing law allowed “impairing, dangerous, untested” compounds in the open market. THC derived from hemp was “being consumed by children” and constituted a “public health problem that we have to address and not wait for another year” in which there could be a “tragic accident.” She suggested new products were essentially “like a chemistry project” and that having overly stringent statutes “freezes” the law, leaving the state unresponsive to “new extraction methods and new products.” Keiser also noted that DOH had been “stretched” by the coronavirus pandemic and had failed to proactively protect consumers from impairing hemp products (audio - 2m, video).
- Rolfes asked Keiser about the differences between her original bill and the proposed substitute. Keiser felt that the biggest change was the definitional focus on cannabinoids “generally [considered to be] or marketed as impairing.” As well, she added several elements of SB 5981 like the CCPRO panel, and new ideas such as the WSLCB stakeholder advisory committee which would “meet at least quarterly.” She pointed to the incorporation of the 20:1 ratio as another change responsive to hemp industry concerns. Rolfes followed up to confirm how she’d addressed the “definition of impairing concern that we heard.” Keiser replied that it had been responded to through “new language” (audio - 1m, video).
- Keiser sought clarification on differences in allowable THC content per serving under the proposed substitute, arguing that her language limited serving size and the total amount in a package, further arguing that some items didn’t have any THC but were still impairing (audio - 1m, video).
- Rivers claimed that LCB had “convened a work group” composed of “well respected scientists to come up with a definition of impairing, and then chose not to use the definition.” She further took issue with the packaging limit concern raised by Keiser, saying it was “incorrect” and insisting that her language also covered cannabinoid content for “the total package” (audio - 1m, video).
- Although WSLCB did convene several scientific experts to inform their work, including two deliberative dialogue sessions in 2021, Cannabis Observer hasn’t seen any evidence that agency leaders directly tasked this group with setting a definition for impairing that the board then rejected.
- To the contrary, it’s Cannabis Observer’s understanding that three of the researchers invited by WSLCB to sit on the deliberative dialogue panels independently connected to hash out a definition of impairing cannabinoids grounded in chemistry language. Those researchers then connected with Bonny Jo Peterson of the Industrial Hemp Association of Washington (IHEMPAWA) who brought their work to the attention of Vicki Christophersen of the Washington CannaBusiness Association (WACA). The definition developed by the researchers was subsequently incorporated into the first competing cannabinoid regulation bill, SB 5767, and later appeared in SB 5951.
- Rivers claimed that LCB had “convened a work group” composed of “well respected scientists to come up with a definition of impairing, and then chose not to use the definition.” She further took issue with the packaging limit concern raised by Keiser, saying it was “incorrect” and insisting that her language also covered cannabinoid content for “the total package” (audio - 1m, video).
- Conway asked committee staff to talk to DOH personnel about the fiscal consequences of the bill, alleging they were “not in that kind of business” of retail product enforcement. Staff agreed to get back to him (audio - 1m, video).
- Amendment A (S-5402.2.) - Rivers said the genesis of her proposed substitute was SB 5981, which she’d co-sponsored, noting it hadn’t been granted a hearing in the WA Senate LCTA. She observed that during public testimony on the bill, most everyone had voiced concern about products being sold outside of the licensed cannabis market and wanted decisions “made in science.” Rivers indicated she had found stakeholder engagement lacking “after [the WSLCB request bill on the topic, HB 1668] died” on the House floor. Not wanting to continue the “controversy” of giving “unfettered rulemaking” power to WSLCB and other things requested in their bill, she decided to take “out all of the disagreeable stuff” (audio - 2m, video).
- After returning from caucus, committee members voted to adopt the proposed substitute by Senator Ann Rivers with no discussion and only a few dissenting votes, then recommended the bill to the Washington State Senate Rules Committee (WA Senate RULE) for calendaring on the floor.
- Vice Chair June Robinson moved that SB 5983 be passed out of committee (audio - <1m, video). She then moved for Rivers’ proposed substitute to be incorporated into SB 5983. There were no remarks offered (audio - <1m, video).
- Rivers’ proposed substitute was adopted by a voice vote of the committee (audio - 1m, video). As Keiser’s amendment was then out of order, Robinson moved for the committee to recommend passage of the amended bill (audio - <1m, video).
- Keiser spoke up before the bill vote, saying she planned to support the bill, as it was “really important” to deal with. However, she reminded colleagues that “the Washington Association of Sheriffs and Police Chiefs did not support this approach, the Washington Traffic Safety Commission did not support this approach, the Washington Public Health Association did not support this approach, the Public Health Roundtable did not support this approach, and the Washington Association for Substance Abuse and Violence Prevention did not support this approach, because it doesn’t provide for standardized, statewide enforcement.” She was fearful that unregulated cannabinoid items would remain for sale, though she acknowledged it was positive that they may be consumed “by not as many people” (audio - 1m, video).
- The entire committee voted in favor of the amended language, except for Senators Keiser, Conway, and Manka Dhingra. Rolfes confirmed SB 5983 would be referred to WA Senate RULE (audio - 2m, video).
- For SB 5983 to become law, there remained several steps lawmakers would need to traverse before adjournment of the session, known as sine die, on Thursday March 10th.
- The next step would entail moving the bill out of WA Senate RULE during a committee meeting or via a chamber pull by senators from the floor. WA Senate RULE committee members had a limited number of “pulls” to move bills to a floor calendar for a second reading with the potential for amendment, followed by a third reading and vote for final passage.
- Keiser, also WA Senate President Pro Tempore, would be closely involved in her caucus’ floor actions.
- Following introduction and referral to a TBD house committee, another public hearing and executive session would be necessary.
- As the bill had been deemed necessary to implement budgets (NTIB), the Washington State House Appropriations Committee (WA House APP) was a likely venue.
- HB 1668 was narrowly passed by that body on February 5th.
- The bill would then need to be calendared by the Washington State House Rules Committee (WA House RUL) or be incorporated into a chamber pull followed by another second and third reading process with the chance for amendments.
- Representative Shelly Kloba, the prime sponsor of HB 1668 and HB 2122, had an opinion piece published on Monday regarding the need to close the cannabinoid “loophole” and may propose further changes to the bill.
- However, so could Representative Drew MacEwen, who offered more than a dozen “hostile amendments” on HB 1668 which contributed to the demise of that bill.
- If the bill were changed by the house, then senators would need to undertake a concurrence or dispute vote in the WA Senate.
- Should SB 5983 pass this point, the now enrolled bill would be delivered to Governor Jay Inslee, who could sign or partially veto legislation before it becomes law, or veto it altogether within 20 days of the end of the session.
- During the WA Senate WM public hearing on SB 5983, WA Governor Senior Policy Advisor Sheri Sawyer testified to convey the concerns of Governor Jay Inslee that the bill was “imperative to averting the serious health impacts that we’re starting to see across the country from converting hemp-derived CBD into delta-8, delta-9-THC or any other impairing cannabinoid" items. Sawyer considered it significant that so many cannabis trade associations backed the bill along with advocates “from public health, and public safety - urging you to take action” (audio - 1m, video).
- The next step would entail moving the bill out of WA Senate RULE during a committee meeting or via a chamber pull by senators from the floor. WA Senate RULE committee members had a limited number of “pulls” to move bills to a floor calendar for a second reading with the potential for amendment, followed by a third reading and vote for final passage.