The task force received an overview of cannabis extraction and product formulation processes and heard status reports from two work groups as an October 31st deadline drew near.
Here are some observations from the Wednesday October 5th Washington State Hemp in Food Task Force (WA Hemp in Food Task Force) Meeting.
My top 4 takeaways:
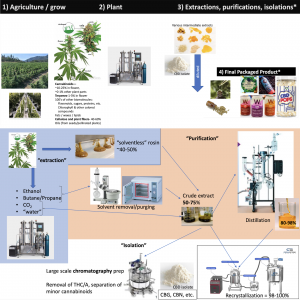
- Medicine Creek Analytics Science Director Amber Wise gave a brief presentation on cannabinoid extraction, purification, and distillation processes before describing dilution of resulting substances into end products (audio - 8m, presentation).
- Wise said interactions on the task force led her “to believe there might be just a little bits of misunderstanding here and there amongst some various folks and I just wanted to do just [a] really, really fast overview and reminder of how some of these molecules are generated, and extraction, and all that kind of stuff.” She would show visual aids since “there are lots and lots of ways to do all of these things…probably 50 ways to do each one of these different steps I'm talking about.”
- Regarding production, Wise clarified “we’re talking about hemp, we're talking about outdoor grows.”
- The numbers in the presentation referred to “a weight percent” with the plant cannabinoids “generally ten to 25% in the flower part, zero to maybe one percent at most in other parts of the plant.” Additionally, “there's terpenes mostly in the flower, there's lots and lots of other biomolecules throughout the plant itself, and cellulose and plant fibers make up 40 to sometimes 70% of the plant weight.”
- There were several “different things that constitute extraction” with outputs that would be considered an “intermediate extraction,” she said. Wise elaborated that after “extractions and purifications that occur, and there's lots and lots of different intermediates,” then companies “dilute these intermediates, greatly most of the time, into” final products.
- This extraction stage was “what generally chemists called the ‘purification’ part, and then what chemists call the ‘isolation’ part.” Solvents such as ethanol, butane/propane, carbon dioxide (CO2), or water “are the main ones” used in extraction. However, with “hydrocarbons solvents…there's an extra step of solvent removal before you get to the crude extract part.” These extracts ranged between 50-75% cannabinoids while the “solventless extractions where we get kief or we press rosin” led to 40-50% cannabinoids by weight.
- Wise remarked that crude extracts could be used in final products, or undergo “distillation,” further purifying them to “between 80 and 90-ish, 90, upper 90s percentage purity of the cannabinoids in there.” By putting this distillate through preparatory chromatography, Wise stated it was possible to remove tetrahydrocannabinol (THC) or “isolate other cannabinoids from a mixture” to get “purified [cannabigerol] CBG, [cannabinol] CBN, other kinds of things like that.” Additionally, “recrystallization…will result in what we referred to as [cannabidiol] CBD isolate, which is 99-100% pure.”
- Considering “the general concentrations of these cannabinoids in the plant material then as we extract and purify they get more and more concentrated, but then we have these large dilution events.” With a “little bit of math…the amounts of weight percent of these concentrates or extracts that are used in these final products…are very, very low”:
- 10 milligram (mg) soda can = 0.003% cannabinoid weight by volume (Wt/v)
- 100mg soda bottle = 0.03% Wt/v
- 10mg/38 gram (g) gummy = 0.026%
- 1000mg/1 ounce (oz) tincture = 3% Wt/v
- 25mg/1g capsule = 2.5%
- Wise concluded with the hope that her presentation could help “visualize some things for folks” as they moved forward.
- University of Washington Center for Cannabis Research (UW CCR) Director Nephi Stella appreciated the information from Wise on cannabinoids by volume, saying “the way I think about it is the amount of cannabinoid that will be ingested.” He felt that 100mg “in soda bottle, at least for CBD we're definitely getting into the medical properties there” (audio - 2m).
- Wise agreed, wanting “to put some value so they weren't all just 10 milligrams.”
- Jessica Tonani, Verda Bio CEO, found the presentation provided a useful perspective “when we think about where we're going to test for especially, like, pesticides, heavy metals, residual solvents” in products.
- The Definitions Work Group provided an update on their recommendations, leading to questions from task force members.
- Draft recommendations from the group were last discussed on September 29th.
- Joy Beckerman, Hemp Ace International Founder and Colorado Hemp Works Senior Advisor and Co-Founder, explained that she and Industrial Hemp Association of Washington (IHEMPAWA) Executive Director Bonny Jo Peterson “were the only ones from definitions that met yesterday,” and hadn't “advance[d] the conversation terribly much” as their opinion “certainly wasn't enough for…a group decision.” They stood by previous recommendations drafted, Beckerman stated, but “we're gonna start with class A being the cannabinoids that do not have the potential to…induce intoxication, and Class B will be the ones that do have the potential to induce intoxication.” This would be “switching up which set of cannabinoids are Class A and Class B” (audio - 5m).
- Peterson said the original order was a response to Washington State Liquor and Cannabis Board (WSLCB) request legislation in the 2022 legislative session, whereas the task force was “working on the opposite spectrum. We're working in the WSDA and Department of Health (DOH) spectrum where…their mission as well as their regulatory style is the complete and utter opposite of LCB.” Anticipating the task force would back legislation involving those agencies “rather than the LCB, that leading with what we are regulating, which is what does not get you high” made the most sense to Peterson, but she recognized “that would be a question…for the broader group.”
- Wise was amenable to the change, but Peterson stressed that the task force would be leading on it as no such classification for cannabinoids had been approved in law or rule.
- Tonani clarified that, in 2023, WSLCB leaders were “essentially proposing all THCs with a little bit modification” and “more wordsmithing than that, but…that is their proposal for these impairing compounds.”
- Beckerman raised the topic of defining ‘hemp extract’ (audio - 4m):
- The draft definition, adapted from language used by the American Herbal Products Association (AHPA), specified “a complex, multicomponent mixture obtained after using a solvent to dissolve components of the biomass. Extracts may be in dry, liquid, or semisolid form. Hemp extracts are not the same as expressed juices or oils, or pure chemicals isolated from an herb, and do not include :
- (i) synthetically modified plant constituents; [*Notes: (1) We will either define “synthetically modified plant constituent” or a similar term and whatever term in this regard is defined will be the term used here to describe this exclusion; (2) there is a thoughtful proposal to include some examples to this exclusion for clarity, such as “…including but not limited to delta-8 tetrahydrocannabinol, tetrahydrocannabinol-O-acetate, hexahydrocannabinol, and tetrahydrocannabiphorol]
- (ii) any food, food ingredient or food additive that is generally recognized as safe pursuant to federal law; or
- (iii) any extract derived from hemp that is not used for human consumption.”
- The draft definition, adapted from language used by the American Herbal Products Association (AHPA), specified “a complex, multicomponent mixture obtained after using a solvent to dissolve components of the biomass. Extracts may be in dry, liquid, or semisolid form. Hemp extracts are not the same as expressed juices or oils, or pure chemicals isolated from an herb, and do not include :
- Tonani explained that the Concentration and Safety Work Group added a recommendation regarding “what hemp products are allowed to include, not only the hemp extract that we discussed last week, but other words like ‘liquid,’ ‘solid,’ ‘semi-solid concentrates,’ ‘isolates’,” and others.
- New recommendation: (rules action) Hemp products allowed in food and dietary supplements include: raw plant material (fresh or dried), juices and expressed liquids, extracts, concentrates, isolates, full and broad-spectrum extracts in liquid, solid or semi-solid form. Hemp products allowed in food and dietary supplements do not include:
- (i) synthetically modified plant constituents;
- (ii) any food, food ingredient or food additive that is generally recognized as safe pursuant to federal law; or
- (iii) any extract derived from hemp that is not used for human consumption.
- New recommendation: (rules action) Hemp products allowed in food and dietary supplements include: raw plant material (fresh or dried), juices and expressed liquids, extracts, concentrates, isolates, full and broad-spectrum extracts in liquid, solid or semi-solid form. Hemp products allowed in food and dietary supplements do not include:
- Beckerman felt that “hemp extract was never intended to describe the broad category of products” to be regulated and she continued to believe “it's important that we have the ‘this does not include’” criteria as part of the definition.
- Peterson stressed that there was an existing definition for hemp extract created as part of a hemp certification statute adopted in 2021 and that any legislation emerging due to their recommendations would need to conform to, or modify, the existing statutes.
- At publication time, RCW 69.07.010(12) defined ‘Hemp extract’ as “a substance or compound intended for human ingestion that is derived from, or made by, processing hemp. The term does not include hemp seeds or hemp seed-derived ingredients that are generally recognized as safe by the United States food and drug administration.”
- Looking over the existing definition in law, Tonani requested “just putting the synthetic exclusion in as well.”
- Returning to the AHPA definition, Wise remarked the “solution to my hesitation with that definition” was to “align with what's already out there” (audio - 14m).
- Beckerman said “we were going to remove that whole part of that sentence” at the end of the AHPA definition and instead “start with ‘this does not include’” and list exceptions in the definition.
- Peterson relayed that others on the task force and in the hemp industry had confided in her that the “solvent end of things still causes quite a bit of concern” with their proposed definition and how “the CO2 industry…the time and pressure end of things…feel that…they would be excluded.”
- Rather than “synthetically modified plant constituents,” Beckerman expected in their recommendation “it's probably gonna be ‘artificial cannabinoid’ or ‘synthesized cannabinoid.’”
- She told the group that task force member Lukas Barfield, Quality West Cannabis (QWC) Owner, had called for giving examples of approved extraction methods in the definition prefaced with “including but not limited to,” so anyone could understand “Washington is not down with those.”
- Wyckoff Farms CEO Dave Wyckoff felt CO2 extraction needed to be explicitly listed because there remained "parts of the industry" that "don't consider CO2 to be a solvent" and using it as a definition example could help.
- Wyckoff later reiterated his belief that “supercritical CO2” needed to be an explicit example of a solvent extraction, leading Wise to ask if there was a jurisdiction that banned the practice; Beckerman wasn't aware of any.
- Eric Elgar, Nextraction Vice President of Quality Operations asked about kief and Wise responded that “will fall under this new recommendation where we list all of these various hemp products that are allowed including “raw plant material fresh, or dried juices, and expressed liquids extracts, concentrates, isolates, full and broad spectrum extracts in liquid, solid, or semi-solid form.” Furthermore, the language in “recommendation (1)(c)” stated “the appropriate state regulatory body will outline processor requirements and restrictions including any relevant testing methods consistent with processing methods.”
- Elgar still wanted “a more simplified way to define an extract that would encompass all this.” Beckerman didn’t think the definition of extract had to directly mention all methods or products allowed, as there would be “a definition for either ‘cannabinoid product’ or ‘hemp cannabinoid product.’”
- Wise considered it to be “unfortunate that hemp extract was the very first definition that was discussed because I feel like people are getting bogged down in trying to make this definition the only thing allowed” rather than “part of this list of allowed things.”
- Wyckoff inquired about products that were generally recognized as safe (GRAS) by federal officials and whether that designation could impact items they were recommending be allowed to be sold (audio - 4m).
- Concluding her remarks for the work group, Beckerman said the AHPA extract definition for dietary supplements was guiding work group thinking, but would not be the final definition itself. WA Hemp in Food Task Force facilitator Steven Byers asked “if we can let this sit for a little while. It sounds like we're really pretty close” (audio - 3m).
- Concentration and Safety Work Group members reviewed alterations to eight proposed recommendations with discussion covering dietary supplements, allowed methods, maximum cannabinoid amounts, and the quickest way to get a state program implemented.
- Ideas from work group members were last discussed on September 29th.
- Wise talked about how WSLCB published an October 3rd bulletin seeking members for a Cannabinoid Science Work Group with expertise in “dietary supplements” among other fields. She wasn’t sure the task force would be addressing supplements in their recommendations, but WSDA Policy Advisor to the Director and Legislative Liaison Kelly McLain said it was fine to include “non-THC” supplements as they were “a huge part of the industry right now” (audio - 2m).
- WSLCB staff planned to continue accepting membership applications for the new work group through Monday October 24th.
- Tonani reviewed “the edits of the recommendations from last week” along with a new recommendation drafted in “another subgroup meeting” following the last task force meeting (audio - 2m).
- The first recommendation only had “a little bit of clarification on text,” but she said members had discussed “testing or requirements may be specific to an extraction method.” Their proposal stated that “the appropriate state regulatory body will outline processor requirements and restrictions including any relevant testing methods consistent with the processing method.”
- A second recommendation mentioned by Tonani revolved around “two categories: the first category once again, would be food; the second category would be dietary supplements.”
- Elgar thought a dietary supplement definition was already in place, and Beckerman and McLain were confident it was part of the state tax code. DOH Deputy Director for Policy and Legislative Relations Rob Oliver told the group he’d pass questions on to Policy Director for Environmental Public Health Joe Laxson. McLain confirmed their recommendation could reference a definition in the tax code for regulations outside of it, but “if you're going to deviate from the definition and the tax statute, then we would want to fully define it here.” Beckerman expected that both the Definitions and Safety work groups could cite the established definition where possible (audio - 4m).
- Contemplating extraction methods that could be allowed, Wyckoff was curious about the WSDA/DOH rulemaking process, particularly whether regulators would “get into all of these these variables, or would you rather see at least a threshold set of standards for these provisions” adapted from other jurisdictions (audio - 11m).
- McLain felt that if a hemp in food law was passed by lawmakers in 2023, it was unlikely to take effect until “sometime around the end of July or beginning of August” of that year. From there, she believed WSDA leaders would want a law explicitly giving rulemaking authorization for specific topics to each agency, and directing them with “significant sideboards, or if you're directing the agencies to use information from other states versus doing…a third party review and having a study done.” These types of details impacted the timeline for rule implementation, according to McLain. She noted expedited rulemaking authority “has to be explicit” and could speed up the process. “We were able to establish a hemp program before most other states in the nation were, because we had expedited rulemaking authority,” she commented. Implementing a system used elsewhere could justify expedited rulemaking, she speculated, “to prevent further injury…or harm to the industry,” but expected that “regardless of pathway, [we’re] looking at like a year from December” before a hemp in food program could be active.
- Wyckoff asked about setting "initial standards" in law “so that there doesn't need to be further regulatory hearings before the legislation is truly effective and people can start to, to engage in this commercial activity.” McLain acknowledged that if left entirely to WSDA to implement it would take longer compared to setting “base standards relatively conservatively in statute and then you direct the agencies via rulemaking to go back and use the science to evaluate and make changes to those.” However, she anticipated that was “relatively difficult, and usually requires, if the numbers are set in statute and we realize later that the numbers need to be higher or lower, we still have to go back and make statute changes which are really difficult.” Generally, “if it lives in statute, it has to be changed in statute,” McLain cautioned.
- Tonani said “we have this November to December to potentially drill down some of these rule recommendations, and I don't know if it would it be helpful from an agency perspective that we could actually like roll up what…expedited rules may look like.” She pushed for settling on the “meat of the recommendations” before the end of October and fleshing out more details ahead of 2023. On extraction, Tonani mentioned conversing with DOH representatives around “setting standards that are allowed potentially, and then allowing anything that is currently allowed” in other food processing in the state like CO2 “and so we may be able to borrow from some other regulations if we look at this as food, especially when we think of the dilution, and just set the limits of allowance.” She believed that “the main concern we have with extraction methods are we just don't want to see the proliferation of…synthesis methods.”
- McLain thought it best for regulators to be set up in law to be nimble and adapt rules, stating “any single one of you could petition for us to quickly start making rule changes if the program that's in play isn't working” whereas values set in statute could leave hemp stakeholders waiting years for legislative action.
- Beckerman returned to the first recommendation, requesting removal of a mention of “non-GRAS food” products. She also pointed out an important hurdle in RCW 15.140.040(5), which stated WSDA “shall regulate the processing of hemp for food products, that are allowable under federal law, in the same manner as other food processing” calling this “the piece in Washington law right now that…makes it so that technically these products are not legal in this state” (audio - 2m).
- Peterson asked McLain about “setting some initial amounts to start with and then saying ‘or as determined in rules.’” McLain agreed that was a possibility, but remarked that bill language would need to be reviewed by WSDA counsel before she could answer definitively. Washington State Department of Agriculture (WSDA) Commodity Inspection Division Assistant Director Jessica Allenton agreed this approach was possible, but needed “a time limit.” Her example was “we initially started the hemp program…the pilot program [was] going to continue until” a “regular program [could] be up and running” (audio - 4m).
- Stella sought to distinguish how this would work differently than a legislative sunset provision (audio - 1m).
- Wyckoff returned to the wording of a sunset provision, “if the federal law came out but was very, very restricting, and if state laws continued to be used and followed and not objected to by the FDA, then this could result in Washington being in a disadvantage.” Wise indicated their language would say “may,” and allowed for flexibility but Beckerman encouraged having legislative staff review the impact of the term (audio - 3m).
- In April 2019, the law implementing the hemp program took immediate effect and required expedited rulemaking. WSDA announced the program rules in November of that year.
- On recommendation 3, Tonani reiterated the plan to make “a base recommendation and clarify it in the November-December time frame” to allow hemp food and supplements “as long as they meet certain safety requirements…So, we don't actually have hard numbers in this but the idea is that there will be certain serving and/or package limits on CBD and then other cannabinoids.” She said this would be based on a table WSDA staff was compiling on standards in place in other jurisdictions “and some of the scientific data out there.” Tonani mentioned “there was one major add and that was the limits on non-impairing cannabinoids outside of CBD” as well as “some discussion around limiting concentrations of other cannabinoids at a per package level” (audio - 2m).
- Beckerman asked Stella about placing a cap on cannabinoids in products “so we don't have…some gigantic gaping hole open for somebody to put 10,000 milligrams of CBG into a product.” Stella liked “the fact that actually we will be revisiting those numbers, you know from time to time…I'm hoping that with that table that we're gonna receive we will be able to actually to aim for a pretty good amount” (audio - 1m).
- Peterson wanted a sense of "how is this tested for,” as “there's multiple other pieces to the whole puzzle of the hemp plant than just cannabinoids.” Beckerman figured that because there were “not a bunch of public health concerns” around plant constituents outside of cannabinoids they shouldn’t focus on developing recommendations to regulate them. Stella stated that the cannabinoids the hemp plant could produce “in significant amounts” were “a handful, or 10 or 20 of those” phytocannabinoids “that we actually understand currently.” Going forward he thought testing should focus on these, and “everybody who wants to add another type of phytocannabinoid, then they actually need to provide the evidence” (audio - 6m).
- Recommendation 4 dealt with setting action limits “for contaminants in different product categories including hemp plant” as a raw ingredient along with “hemp extracts used in food and dietary supplements,” Tonani said. She credited a DOH staffer with suggesting that companies be permitted to test their intermediate or end products, while DOH conducted testing “randomly” of end products only. Tonani described how work group members got on board with the idea of safeguarding consumer health, “but there is this dilution issue which is what Amber presented, and if you look at the slides that she presented…the concentration of the hemp material that's actually going to end up in” an end product was “fairly low.” Tonani concluded that their goal had been “to look at the hemp ingredient itself and not necessarily regulate the entire food supply chain” (audio - 3m).
- David Gang, Washington State University Center for Cannabis Policy, Research, and Outreach (WSU CCPRO) Director, returned to the conversation after an absence, and Tonani explained that their recommendation involved “actionable levels that the Department of Health puts in place" for contaminants and that WSDA would conduct randomized testing. In the event a product tested “hot for those you better have an ingredient level [certificate of analysis] COA that says that's not coming from the hemp,” she relayed. Gang asked if this was done on products like fruit or “baby food,” and Tonani stated that to her knowledge it wasn’t, which left him concerned they were reaching a “situation where we're regulating the entire food supply chain” for hemp food only (audio - 13m).
- Wise disagreed, as they were only expecting to test for “cannabinoids and microbial at the batch or lot level, and that will probably be included on the label” while “other contaminants will be set depending upon product type.” She continued, saying an end product could be randomly “spot checked…assuming there's a state regulatory body that's interested in doing that and there's funding for that.” Additionally, she didn’t want to “create a new sort of traceability system where you're not allowed to sell something if there isn't a COA attached to it.” She added that companies which didn’t use solvents in their extraction process shouldn’t be required to test for them.
- Gang still felt there wasn’t sufficient specificity on what regulatory body would take on product testing. Tonani conveyed DOH’s practice to “spot check packaged goods for microbials. If…the package is hot for microbials they go in and they audit the facility. And, part of that auditing in the facility is kind of making sure that the company has…done everything that they need to do along the way to hear that that product shouldn't have microbials in it.” She said the idea of doing something similar for hemp in food, “that you as a processor are responsible for auditing that ingredient if they spot check you, your product on the shelf, and it's hot, you pull your COAs and you say it's not hot from the hemp, it's hot from the sugar or something else. But it's your responsibility to kind of keep that back in documentation.”
- Wyckoff understood Gang’s observation, but he thought DOH and FDA oversight of the food industry would assist in keeping hemp in food safe, “as it does for most food ingredients now.” He was confident that between the two agencies, the industry could “take care of a lot of these issues" through "existing adulterant regulations."
- Expecting that the Code of Federal Regulations (CFR) would be “the drum beat of these additional regs because it's not cannabinoids or non-GRAS,” Beckerman thought both the FDA and state officials could “come in at any time and inspect your records” for hemp testing and compare results to “a master manufacturing record that shows that all of these specs and all of these boundaries are met and if you can't prove them…there becomes some type of corrective, hopefully not disciplinary, action.” She talked about the need to ensure COAs connected to the QR code on a product’s label.
- On recommendation 5, Tonani addressed how it would require listing amounts of CBD, along with other cannabinoids classes. The only change from the past draft was “we believe that there should be the amount of cannabinoids actually on the package…and other marketing compounds” on the main informational panel for a package, she said (audio - 1m).
- Beckerman indicated “what should be on the primary display panel is…the names of what the cannabinoids are that you're marketing in that product, the milligrams should actually be on the information panel.” A problem emerged “when we put the milligrams on the primary display panel” she commented, as “you can get in trouble for a label violation because it is confusing to the consumer” and had to be either on “the information panel for the food, and on the supplement facts panel for the supplements.” Tonani thanked Beckerman and promised the language would get “cleaned up” before their final recommendation (audio - 3m).
- Recommendation 6 pertained to compliance with federal law and was unchanged, while comments on recommendation 7 occurred earlier in the task force meeting (audio - 3m).
- Tonani and Beckerman briefly debated whether the recommendations had to use the phrase “hemp cannabinoid product" or whether “it’s implied.”
- WA Hemp in Food Task Force facilitator Steven Byers helped the group plan additional task force meetings before their recommendations would need to be presented to WSDA staff for incorporation into a report due to legislators by December 1st.
- Byers and members agreed to meet on Wednesday October 19th, granting “from now until that time to do more work in the subgroup” as they began “to share stuff with the WSDA” ahead of the final report to the legislature (audio - 2m).
- Allenton previously outlined how task force recommendations would need to be provided to WSDA staff by October 31st.
- Beckerman was curious about the status of WSDA information gathering, saying her dialogue with Jill Wisehart, the WSDA staffer responsible for compiling the data, left her with the impression that she “didn't understand the scope” of their work (audio - 6m).
- Wise offered her impression from McLain that someone was on the job. “I'm trusting that they are doing their job because that's what they're paid for,” Wise said, whereas “I am a volunteer and I am preparing the amount of material that I am willing to prepare” to help the group’s effort.
- Tonani was under the impression that Wisehart was “taking that data along with other data and compiling it in an easy to read table so that…we as a group can go through thousands of pages of documents” .
- Byers promised to get clarification on whom at WSDA was managing the information.
- As consensus grew among members for there to be another meeting before their recommendation deadline, Byers scheduled another meeting for October 26th (audio - 3m).
- Byers and members agreed to meet on Wednesday October 19th, granting “from now until that time to do more work in the subgroup” as they began “to share stuff with the WSDA” ahead of the final report to the legislature (audio - 2m).