Several task force members helped develop the scope of a third-party contract to review scientific literature and contemplated a legislative strategy for pursuit in 2023.
Here are some observations from the Wednesday December 21st Washington State Hemp in Food Task Force (WA Hemp in Food Task Force) Meeting.
My top 3 takeaways:
- Jill Wisehart, Washington State Department of Agriculture (WSDA) Produce Safety Program Management Analyst, mentioned the group’s legislative report and a review of scientific literature around tetrahydrocannabinol (THC) content to prompt follow up from task force members.
- At the group’s meeting a week earlier on December 14th, members sounded resolved to make another attempt to reach consensus on a THC content limit for hemp in food and dietary supplements for draft legislation which some members expressed interest in.
- Wisehart said she’d “received several” suggested edits to the final report submitted on December 1st and asked if more would be coming, or if she was “at a point where I could pretty much get the ones that I've received moving forward?” (audio - 1m)
- Bonny Jo Peterson, Industrial Hemp Association of Washington (IHEMPAWA) Executive Director, commented that her priorities had been “one diagram with the hemp extract definition, along with “typo…type stuff.”
- Wisehart assured the group she’d be open to edits through Friday, December 23rd.
- Moving to a review of academic literature, Wisehart reported that “we have plenty of funding left” and would “proceed with doing a contracted literature review.” She noted “we can self-select a direct contract for 30,000 [dollars], and we have [$]40,000 if it's a women or minority-owned business” (audio - 6m).
- Verda Bio CEO Jessica Tonani read through the initial draft of questions they wanted the contractor to address before Medicine Creek AnalyticsScience DirectorAmber Wise shared revised wording:
- “1) What is the amount (in mg [milligrams]) of impairment for THC in healthy adults, non-cannabis users? How long does that impairment last for? How far apart should the ingestion be to avoid escalating effects on impairment?
- 2) What studies assess health and cognitive risks for sensitive populations (i.e., youth and [immunosuppressed]) when consuming <2.5 mg of THC, <1 mg of THC, or trace amounts of THC?
- 3) What studies assess the safety of full spectrum products in a consumer product?”
- Spoke Sciences Chief Regulatory Officer and Vice President of Chemistry Brad Douglass called for giving prospective vendors more context, how “these questions were topics of discussion amongst the task force.” He suggested they speak with task force members or staff directly so they weren’t “starting from scratch.”
- Peterson expected studies cited in their final report would accompany the contract. Wisehart pointed out that she hadn’t prepared for that, and backed Douglass’ idea of having members speak to any eventual contractor in a type of “hand off” of what had been reviewed. Peterson sought additional context on whether “scientists” would conduct the review, and Wisehart promised to double check the contract wording.
- Verda Bio CEO Jessica Tonani read through the initial draft of questions they wanted the contractor to address before Medicine Creek AnalyticsScience DirectorAmber Wise shared revised wording:
- Later in the meeting, Douglass asked how the contract would be fulfilled. Wisehart responded that Washington State had a “master contract list and so you can do a direct contract that way and then there's a purchasing threshold that we have that you can just direct buy, or direct contract.” Adding criteria to what they wanted, she would “see if anyone fits that; I'd prioritize minority and women-owned businesses, because then the amount bumps up” (audio - 3m).
- Peterson explained that a toxicologist she knew, Marilyn Huestis, was on that list and had “helped Canada with…their laws and that report that they had out.” She’d previously relayed to Kelly McLain, WSDA Policy Advisor to the Director and Legislative Liaison, that Huestis hadn’t been available to help the task force. However, Peterson believed “maybe now that things are narrowed to certain studies and certain questions” Huestis might conduct the review.
- With a noted career as a “toxicologist who studied the effects of illicit drugs on the body, brain, and in utero,” Huestis discussed research around detecting cannabinoid impairment while driving in 2017.
- Tonani advised the hiring of Dominic Corva, Center for the Study of Cannabis and Social Policy (CASP) Founder as well as Director of Cannabis Studies at the California State Polytechnic University, Humboldt (Cal Poly Humboldt).
- A former Citizen Observer, at time of publication, Corva also served as a researcher at the Humboldt institute for Interdisciplinary Marijuana Research (HIIMR) and Board Member and Secretary of International Cannabis and Hemp Standards (ICHS).
- Learn more from the Washington State Department of Enterprise Services (WA DES) resource on How to Use Statewide Contracts.
- Peterson explained that a toxicologist she knew, Marilyn Huestis, was on that list and had “helped Canada with…their laws and that report that they had out.” She’d previously relayed to Kelly McLain, WSDA Policy Advisor to the Director and Legislative Liaison, that Huestis hadn’t been available to help the task force. However, Peterson believed “maybe now that things are narrowed to certain studies and certain questions” Huestis might conduct the review.
- Contemplating draft legislation for 2023, several members strategized how to set THC limits for hemp products and explain the rationale in a way legislators and regulators would agree to - and sufficiently fund (audio - 13m).
- When the topic of draft legislation arose, Peterson explained the proposed text was “not something [we] can share yet,” but she would address some of the comments from those who had seen it.
- Wisehart told the group that the WSDA “Food Safety program doesn't have the current staffing capacity to absorb this kind of proposed legislation without” direct budgeting by lawmakers.
- The second issue Wisehart raised was “certification versus a license for processing for hemp extraction…from their point of view it would be simpler to go through the licensing rather than the endorsement of venue.” Peterson explained that certification had been used in 2021 legislation and the law stated “once there's a way to do [licensing] within…the state that that goes away so that there's like a sunset to it, but people would have that license.” She’d asked McLain “do we still need that in there, or could [it] be handled…in a simple way” as she believed staff hadn’t “quite understood...why that was in there.” Peterson doubted a mention of certification was “even necessary” in an eventual bill.
- Another concern of staff, said Wisehart, related to “process authority” and how “they rely on FDA [U.S. Food and Drug Administration], academia, and process authorities in the industry when it comes to determining standards for food safety,” whereas WSDA personnel “don't have the training and resources to evaluate the safety of CBD [cannabidiol] in food, and to set standards.”
- Peterson remarked that when similar hesitations were raised about prior bills, it had been a matter of “how much do we have to specify in the bill of what they need to specifically do.” However, “with the pilot program piece to the whole puzzle [there could be] space for that to be determined.”
- Tonani understood the aim of setting a THC threshold for hemp products was due to food safety staff being able to add a standard to the testing regime they were already responsible for “which they would regulate around.” She also had the impression that officials struggled to fund enforcement on existing hemp products outside of the adult-use cannabis sector, and her opinion was that they “have to bite off that and eliminate the unsafe products and allow some safe products…and I think that if we just continue to say ‘there's no money to regulate these products,’ we're going to continue to have proliferation of unsafe products in the market.” Eric Elgar, Nextraction Vice President of Quality Operations concurred with this take, figuring if the task force members “put all this together, but if there's no money for enforcement on it …we've done all this, but it doesn't change anything necessarily.”
- Petersen agreed the legislation they were considering had a “pretty significant dollar amount,” characterizing the policy as being “more expensive the more we have to do.” She estimated that by the time she requested budget for necessary staff, training, and rulemaking, “guess I have to…ask for two million dollars because there's gonna have to be part of that for enforcement.” Peterson elaborated that in talking with McLain, she’d found that depending on what rules they wanted funding for, a bill “could be up to four” million dollars. Wisehart noted the Food Safety staff had 25 inspectors in the “manufactured foods regulatory program,” and expected “they need quite a bit more staff to absorb this legislation into their program.”
- Elgar thought since established laws verifying hemp facilities included registered producers following applicable good manufacturing practices (GMPs) which “any food…producer is going to have to” comply with, less additional rulemaking would need to be done. Tonani recognized there would always be costs with oversight of hemp commodities, and knew members hadn’t “thought it would come for free.”
- Find out more from opinion and policy documents on cannabis, hemp, and GMPs:
- Cannabis Food Safety - FDA/GMP Compliance in Manufacturing, Dispensaries and Growers (2019)
- Facts About Current Good Manufacturing Practices (cGMPs) And Their Role In The Cannabis Industry (2020)
- GMPs & Cannabis Manufacturing (2021)
- New York state Cannabinoid Hemp Processor GMP Audit Guidance
- A 2021 Safe Food Team presentation to Vermont regulators on Manufacturing of Hemp Products
- U.S. Department of Agriculture (USDA) resources on Good Manufacturing Practices Audits
- Find out more from opinion and policy documents on cannabis, hemp, and GMPs:
- Douglass felt there was less need for legislating new process authorities for WSDA because the task force recommendations would include “giving them the standards that they need…to move forward.” He felt “it's a very small scope which we essentially provided the groundwork for.” Wisehart agreed “the information was there,” but they’d still need new staff and training around it.
- A complication noted by Peterson involved dietary supplements, and whether they would be listed separately in legislation as she understood them to have the same facility regulations as food processing. However, there might be a need to specify the supplements as a “bucket,” she remarked.
- Elgar observed there “actually [was] a difference in the good manufacturing processes that the FDA has between human food and dietary supplements. Human food is…basically a safety driven GMP…sanitation” and “other things.” Meanwhile, he said there was “a big quality aspect to the GMPs that go into dietary supplements that…are quite different” from food processing inspections.
- Peterson added that “they want to handle it like what they do in the state for other dietary supplements and that'll make it a lot simpler for us and that'll save us about $500,000.”
- In Tonani’s view, “what we have put forward on the scientific level” around ratios of CBD to THC and other aspects were “really complicated subjects," and an informational session for lawmakers could be beneficial to help them understand “why those were put in place and what those mean.” She felt there had been “two, three years of a lot of confusion around regulation in the space,” adding members could “get in front of it” by answering questions ahead of time. Wisehart promised to follow up on the possibility with McLain (audio - 8m).
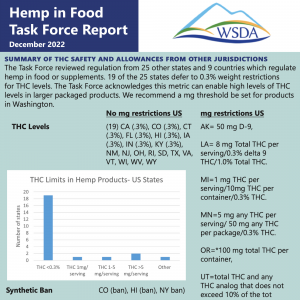
- Peterson proposed an “infographic” on the subject of cannabinoid ratios and their recommendations “and setting up appointments to actually explain it to them”
- Such a panel for elected officials and regulators, on a “meta level or up level, it helps us and everybody keep our eye on the ball,” suggested Douglass. Rather than fixating on a proposed THC limit for hemp products, he thought the event could focus on the “largely unregulated…impairing cannabinoid products out there.”
- Peterson agreed, finding the task force had “done what we set out to do” as mandated by the budget proviso establishing the group. Their recommendations on regulating hemp in food were “really eliminating the main issue," and she called them a “mechanism to say ‘here, these specific chemically transformed cannabinoids,’ or whatever they're making, are not allowed.” Peterson then advised requesting to present to legislative committees once they had a suitable infographic and talking points, or hosting their own remote discussion on the topic.
- Lukas Barfield, Quality West Cannabis (QWC) Owner, wanted this to be done in time for “lobby days” during the legislative session.
- Tonani wanted to see the same outreach occur to cannabis industry organizations to address “misconceptions floating around” on what potential legislation would allow and what cannabinoid ratios were recommended.
- Wisehart mentioned the infographic could be added to the scientific review contract, but a review wouldn’t arrive in a timely manner for the session. Peterson agreed to draft the document, but encouraged adding the document into the contract. Wisehart agreed that adding “deliverables” was “an option” for state contracts.
- A subset of members considered task force logistics during the legislative session, and planned the group’s first meeting of 2023 for Wednesday January 11th.
- Wisehart explained how task force members, rather than WSDA “facilitators,” would set their “agenda and purpose” moving forward. She conveyed McLain’s sentiment that the department wasn’t “the ultimate decision maker in the impairing space,” and their leadership wouldn’t be lobbying for sponsors for any task force legislation (audio - 1m).
- Members discussed when it was best to meet again in 2023, considering the legislative calendar and the timeline for committee activity as the session would begin on January 9th. Wisehart expected the next meeting would be for members intending to be “more intimately involved…with the legislative process” (audio - 7m).
- Members settled on January 11th for their next meeting, and Wisehart indicated she’d be able to “get that contract going soon to get the literature review done” as well as discuss “more specifics on the draft bill at that point as well” (audio - 1m).
Information Set
-
Complete Audio - Cannabis Observer
[ InfoSet ]
-
Audio - Cannabis Observer - 00 - Complete (41m 17s; Dec 21, 2022) [ Info ]
-
Audio - Cannabis Observer - 01 - Welcome - Jill Wisehart (28s; Dec 21, 2022) [ Info ]
-
Audio - Cannabis Observer - 02 - Approval of Agenda - Jill Wisehart (1m 16s; Dec 21, 2022) [ Info ]
-
Audio - Cannabis Observer - 03 - Legislative Report - Jill Wisehart (1m 16s; Dec 21, 2022) [ Info ]
-
Audio - Cannabis Observer - 04 - Task Force Logistics - Jill Wisehart (58s; Dec 21, 2022) [ Info ]
-
Audio - Cannabis Observer - 05 - Task Force Logistics - Question - Timing - Jill Wisehart (6m 48s; Dec 21, 2022) [ Info ]
-
Audio - Cannabis Observer - 06 - Literature Review - Jill Wisehart (5m 52s; Dec 21, 2022) [ Info ]
-
Audio - Cannabis Observer - 07 - Draft Legislation Review - Jill Wisehart (12m 54s; Dec 21, 2022) [ Info ]
-
Audio - Cannabis Observer - 08 - Legislator and Regulator Education - Jessica Tonani (8m 14s; Dec 21, 2022) [ Info ]
-
Audio - Cannabis Observer - 09 - Wrapping Up - Jill Wisehart (9s; Dec 21, 2022) [ Info ]
-
Audio - Cannabis Observer - 10 - Question - Literature Review Contract Fulfillment Process - Brad Douglass (2m 51s; Dec 21, 2022) [ Info ]
-
Audio - Cannabis Observer - 11 - Wrapping Up - Jill Wisehart (31s; Dec 21, 2022) [ Info ]
-
-
WA Hemp in Food Task Force - Meeting - General Information
[ InfoSet ]
-
Announcement - v1 (May 16, 2022) [ Info ]
-
Announcement - v2 (May 18, 2022) [ Info ]
-
Document Repository - WSDA (Sep 6, 2022) [ Info ]
-
Legislative Report - v3 (Nov 30, 2022) [ Info ]
-
Legislative Report - v4 (Jan 12, 2023) [ Info ]
-