A panel of experts discussed the chemistry of the cannabis plant and its pharmacology with a focus on the conversion of CBD into other cannabinoids for commercial products.
Here are some observations from the Thursday June 3rd Washington State Liquor and Cannabis Board (WSLCB) Deliberative Dialogue on Cannabis Plant Chemistry.
My top 3 takeaways:
- WSLCB staff welcomed attendees, laid out parameters for the dialogue as it related to the rulemaking on Tetrahydrocannabinol (THC) Compounds other than Delta-9, and had panelists introduce themselves.
- Reviewing the agenda, Policy and Rules Manager Kathy Hoffman addressed participant roles, guidelines for the dialogue, and noted both the question and answer (Q&A) chat box and the regular chat box attendees would use for the session (audio - 11m).
- She described the session as providing “a platform for panelists to share and discuss:
- plant chemistry perspective;
- increase opportunity for genuine, respectful, moderated dialogue between all participants;
- and begin dialogue that can be used to inform THC compounds other than delta-9 rule and policy development in the future.”
- Hoffman acknowledged the complexity of the subject matter and asked for understanding if responses to the prepared questions ran long. She indicated that although the May 12th announcement for the session included a call for questions, “we did not receive any questions for today’s panel in advance.” Hoffman said the questions had been developed “with our panelists to begin what we hope will be a...meaningful dialogue used to inform our pathway forward.”
- “We are well aware of the positions of industry associations representing licensed cannabis businesses,” Hoffman stated, “but we’re also well aware that interests, or indeed, concern around cannabis plant chemistry extends beyond LCB licensees.” She said the session was “for all voices to be heard” because agency officials valued a “collaborative and transparent process” and wanted all participants to “listen, think, and explore new ideas together.” Hoffman made it clear that panelists were offering expert opinion, and not forming or stating government policy.
- Hoffman went through the timeline that had led to the scheduling of the deliberative dialogue:
- On April 28th, staff announced a policy statement on delta-8-THC and other cannabinoids, sending a clarifying bulletin about the policy statement on May 3rd.
- A rulemaking project on THC Compounds other than Delta-9 was approved by board members on May 12th, a day after a presentation by Hoffman during the May 11th board caucus.
- She described the session as providing “a platform for panelists to share and discuss:
- Policy Affairs and Outreach Director Justin Nordhorn noted cannabis plant chemistry was an “ever changing topic, all across the United States” and that WSLCB leaders were “engaged” with officials in other states and groups like the Cannabis Regulators Association (CANNRA, audio - 3m).
- He mentioned that beyond near-term rulemaking, the session would “inform the LCB, where do we need to go with any type of legislative request and how do we work with stakeholders to accomplish those particular goals?” He noted participants from state and local health agencies, representatives of cities, “and also our colleagues from Canada” were present to help in making “the best reasonable policy that we can.”
- On February 10th, WSLCB contracted with Gillian Schauer, a research scientist at the University of Washington Addictions, Drug & Alcohol Institute (ADAI), formerly the Alcohol & Drug Abuse Institute. Hoffman noted that Schauer was already representing public health issues within CANNRA in November 2020 along with the first public acknowledgment WSLCB was looking at cannabinoid regulation beyond delta-9-THC. On March 16th, Director Rick Garza announced the agency had contracted with Schauer saying she’d “brought the states together” through CANNRA to learn “what are they doing in response to this issue around delta-8.”
- A staffer for the Alberta Gaming, Liquor, and Cannabis Commission (AGLC) participated in a listen and learn forum on June 1st.
- He mentioned that beyond near-term rulemaking, the session would “inform the LCB, where do we need to go with any type of legislative request and how do we work with stakeholders to accomplish those particular goals?” He noted participants from state and local health agencies, representatives of cities, “and also our colleagues from Canada” were present to help in making “the best reasonable policy that we can.”
- WSLCB Chemist Nicholas Poolman, who helped moderate the panel, stated that he liaised between accredited cannabis testing laboratories, succinctly adding, “I do a lot of the science for the agency” (audio - 1m).
- Poolman was hired by WSLCB in 2019 having formerly worked at Capitol Analysis as well as the Washington State Department of Agriculture (WSDA) lab responsible for analyzing WSLCB cannabis samples. At publication time, he helped represent the agency on the Washington State Department of Ecology Cannabis Science Task Force (CSTF) as a non-voting Steering Committee member.
- Also supporting the session for WSLCB:
- Audrey Vasek, Policy and Rules Coordinator
- Jeff Kildahl, Policy and Rules Coordinator
- Kendra Hodgson, Marijuana Examiners Manager
- At publication time, Hodgson served as the voting WSLCB representative on the CSTF Steering Committee.
- Tierney Hamilton-Steele, Administrative Assistant
- The guest experts invited for the panel:
- Jessica Tonani, VerdaBio CEO (audio - 1m)
- VerdaBio was “the first group in the state of Washington to get a cannabis research license,” and had worked with “a number of companies, universities, and even federal labs,” Tonani commented. She described her work on “the genomics of cannabis, including releasing a couple of cannabis genomes, and genetics of cannabis dictate some cannabinoid synthesis, but not all.”
- Brad Douglass, The Werc Shop Vice President of Intellectual Property & Regulatory Affairs (audio - 1m)
- With a background in “organic medicinal chemistry...I have over a decade of experience,” Douglass stated, working with the U.S. Food and Drug Administration (FDA) as an affairs consultant on “food and dietary ingredient legality.” He also had experience with how FDA and “other global regulators deal with synthetics.” Douglass joined “the cannabis industry over about a decade ago” at the Werc Shop where he’d served as lab director as well as handling “extracting, purifying, and manufacturing cannabinoids and cannabis products.”
- David Gang, Washington State University (WSU) Professor at the Institute of Biological Chemistry (audio - 2m)
- Gang offered his experience as “a plant biochemist/chemist” researching medicinal plants, saying he was a former president of the Phytochemical Society of North America (PSNA) which was an association of researchers “interested in the chemistry of plants.” Additionally, he served as Assistant Director of the Agricultural Research Center for the WSU Office of Research where he was a “point person for topics related to cannabis...as well as research related to hemp.” Gang noted that federal law had made research on cannabis cumbersome while hemp research had become much easier. He believed the dialogue would be interesting, in part, because “it’s really the same species, it’s just been differentiated into different types and the chemistry that we’re talking about today is actually pretty important for that.”
- Nephi Stella, University of Washington (UW) Pharmacology Professor (audio - 1m)
- Having studied cannabinoid research “for about 25 years,” Stella said he’d watched from “the early days and, and the legalization” and looked at “how cannabinoids will affect our body, our brain.” He was centrally concerned with “trying to understand and optimize the medical properties of cannabis” which necessitated learning about possible “toxicity or side effects.”
- Check out Stella’s September 2019 presentation on cannabis research to lawmakers. He was also a cosigner of the 2020 consensus statement on Cannabis Concentration and Health Risks: A Report for the Washington State Prevention Research Subcommittee (PRSC).
- Amber Wise, Medicine Creek Analytics Science Director (audio - 1m)
- Wise described having a PhD in chemistry and prior experience as a professor at “undergrad[uate] institutions for a number of years” who had researched “toxicology and public health.” Owing to her work in the cannabis testing sector, she intended to “shed some light on some of the nuances behind analytics and detection” in cannabis chemistry.
- At publication time, Wise was also a Steering Committee member on the CSTF.
- Wise described having a PhD in chemistry and prior experience as a professor at “undergrad[uate] institutions for a number of years” who had researched “toxicology and public health.” Owing to her work in the cannabis testing sector, she intended to “shed some light on some of the nuances behind analytics and detection” in cannabis chemistry.
- Jessica Tonani, VerdaBio CEO (audio - 1m)
- Reviewing the agenda, Policy and Rules Manager Kathy Hoffman addressed participant roles, guidelines for the dialogue, and noted both the question and answer (Q&A) chat box and the regular chat box attendees would use for the session (audio - 11m).
- Panelists responded to five questions prepared with agency representatives to establish fundamentals of cannabis plant chemistry, specifically around the processes for converting hemp biomass into psychotropic cannabinoids for use in cannabis products.
- Question 1 -“How do the effects of delta-8 THC differ from those of delta-9 THC (and other THC isomers)? Does it matter if the compound is produced in plants or chemically?” (audio - <1m)
- Stella’s work in pharmacology had him study “the molecule, then I think about how it travels in our body and then how does it affect the brain or our body.” This involved evaluating methods of ingestion, dosages, and “formulations” needed to achieve certain effects, and ultimately understanding substances “at the molecular level.” He went through several points he considered relevant to the question (audio - 9m):
- With delta-9-THC being “the main cannabinoid compound that has been studied,” Stella suggested this was the “molecule that has been legalized, and produces some effects on our body that can be differentiated on the brain, or on the entire body.” He commented that since around 2006 researchers have looked more closely at cannabidiol (CBD), “or even mixtures of delta-9 and cannabidiols together” to look at what “biological response these molecules will produce.” Stella said studies to date showed “very different biological responses because they work through very different mechanisms.”
- Stella talked about the human endocannabinoid system which includes receptors in the body with proteins that “interact” with cannabinoid molecules “just like a key lock, it will open up some biological response, either in the brain...or in the body.”
- As to sources of cannabinoids, Stella noted “phytocannabinoids produced by the plant” and then extracted, “exactly the same molecule can be synthesized in the laboratory, or one could actually invent a new molecule.” Regardless of the source, he told the group “if the molecule is a 100% pure, either from the plant or the lab...in our perspective, this molecule will produce the same biological effect.”
- Stella talked about looking at the “side effects” of delta-9-THC “particularly in the context of the vulnerable populations” like teenagers, whose bodies “really might actually have some detrimental effects from taking, regularly, delta-9-THC.” CBD had a “different profile,” he said, with “much more medicinal properties.”
- Stella described “peer-reviewed scientific papers that have been published in the field” on delta-8-THC and delta-10-THC. Since the 1960s, “a small number [of], but high quality” research papers had indicated a “small chemical difference” between delta-8-THC and delta-9-THC. He observed that “frankly, the field has not paid much attention” to delta-8-THC and delta-10-THC, aside from a mere “handful of papers that were produced in the 80s that were studying what delta-8 was doing.” The main findings of those studies, Stella said, was “the biological activity of delta-8 is very similar to” the activity of delta-9-THC, though perhaps “more mild” with “maybe a portion of the effect, and maybe [with] the duration a little bit differently.” Because it did “almost the same thing as delta-9,” scientists hadn’t studied delta-8-THC closely, he stated. Stella said he had “a hard time finding” peer-reviewed information about delta-10-THC.
- Take a look at a 1979 research document from the U.S. National Institute on Drug Abuse (NIDA) covering the Structure-Activity Relationships of the Cannabinoids.
- In 2010, Dutch researchers released a report, Chemistry of Cannabis, that found delta-8-THC possessed “a similar pharmacological profile and slightly lower psychoactive potency” than delta-9-THC.
- Though there was “very limited information” on many cannabinoids, Stella felt researchers had better tools to evaluate them than past generations.
- Tonani commented on early research involving pediatric medical patients which may have sought “less psychotropic effect” from cannabis (audio - 1m).
- Gang focused on the source portion of the question, “does it matter if the compound is produced in plants or chemically?” He agreed the same molecule could be produced in a lab or by plants “enzymatically.” Gang said “a certain type of these molecules” were produced by proteins in cannabis, “the right handed version, there’s also a left-handed version that the plant doesn’t make but if you are a chemist making these in a lab” then you could create it. He elaborated that “controlling the difference of being able to make the left or right handed version is very difficult” and that often “you’ll get a mixture of both of those.” He speculated that “one of them will be active, and the other one may have a completely different activity” (audio - 1m).
- Douglass spoke to a distinction between “physiological effects and perhaps psychotropic effects,” calling attention to “what the body does to a molecule,” known as the field of pharmacokinetics. The main processes were “metabolization and excretion,” and he said there had been some studies involving delta-8-THC pharmacokinetics “relative to delta-9-THC, and it’s very similar.” Both compounds were excreted by the body, which mattered for “understanding the safety” of substances, he stated (audio - 1m).
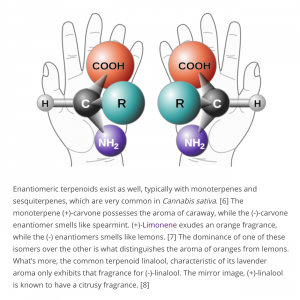
- Poolman talked about delta-9-THC and delta-8-THC enantiomers, explaining that both molecules had “four enantiomers, and we are very specifically talking about one the plant makes” and that with the other three enantiomers “we really know nothing about” them. As the molecules had “two stereocenters,” he elaborated, “essentially, you could have right-right, right-left, left-right, and left-left.” Whereas “the plant, with its enzymes, only makes one set of hands, the other three sets of hands” could be created chemically but “we know nothing about” them because they weren’t present “in the plant” (audio - 4m).
- Wise translated for “non-chemists,” stating that different enantiomers had “different three-dimensional shapes for the molecule.” While chiral compounds could appear similar when drawn, “in three-dimensional space they take up different shapes and that means they bind to our receptors differently and have different activity.”
- Douglass used the example of the terpene carvone which had different smells based on which enantiomer was present.
- Gang compared the cannabinoid receptors in the body to different gloves. While some latex gloves fit either hand, “some work gloves are really designed to go on only on one hand” and the receptors were like the latter. This meant different enantiomers could produce different responses, he explained.
- Stella’s work in pharmacology had him study “the molecule, then I think about how it travels in our body and then how does it affect the brain or our body.” This involved evaluating methods of ingestion, dosages, and “formulations” needed to achieve certain effects, and ultimately understanding substances “at the molecular level.” He went through several points he considered relevant to the question (audio - 9m):
- Question 2 - “When producing delta-8 THC, what do and don't we know about byproducts? How are these detected?” (audio - <1m)
- Wise described the conversion of CBD into types of THC. “There are different steps along the pathway,” she said, including the most economical and “highly used transformation at the moment which is, you know, converting CBD into delta-8” (audio - 6m):
- First, “you have to get the CBD out of the plant material,” Wise told the group, as a “crude” extract which may be further distilled and isolated. The conversion process took different forms, she said, ranging from “relatively safe and clean chemistries to things that I don’t even want to mention here that I’ve seen” mentioned online. Following the conversion, Wise added that “you need to generally do some sort of cleanup on the finished product.”
- The byproducts from processes fell into two categories for Wise, one being “side reactions and chemistry that didn’t go exactly as you think it does” because methods were never 100% complete. The source material used and processes undertaken produced varying side reactions creating “all kind of other organic molecules that aren’t necessarily cannabinoids,” she observed. The other byproduct type was “leftovers from the chemistry steps, the residual solvents, the catalysts,” Wise said, raising questions about whether these substances were “removed from the final product.”
- Turning to testing, Wise said that many commercial cannabis labs lacked the “ability to identify or separate many” cannabinoid compounds. For the “side reaction chemistries,” she communicated that some had “the same molecular weight” and while it was possible to separate and identify them, it was costly, “very time consuming, and there aren’t a lot of standardized methods or guidance for us to go on.” Wise relayed that a testing standard for delta-10-THC “just became commercially available” and while some labs in the state could test for it, it took time to develop a testing methods. “If you're not doing your separation very well, you can get these molecules [to] come out together” she told the group, meaning material “that looks like delta-8 might actually be a combination of delta-9 or some other side reaction products.”
- Wise summarized by saying there were “a number of different ways to go from the starting plant material to a final product.” She hoped “many people are potentially doing this in a clean and safe and correct way using trained chemists.” However, actors in the unregulated cannabis market could be converting CBD “in garages and using internet forums as instructions, and we’re getting god-knows-what out as a product.”
- Tonani likened the process to “cooking eggs" as people have different skills and facilities for scrambling eggs. The same goes for the conversion of cannabinoids, and chemists would have “different quality in their production.” Wise noted that the way CBD was extracted would have an effect on the conversion process and lead to “different...groups of dominant molecules.” How “crude” or “pure” the CBD input was had an important effect on the byproducts in the result, she stressed, and when converting the substance “every time you do a step there’s like multiple pathways that you can take.” Wise added that a “government lab had ways that you could...detect the byproducts...and figure out the chemistry they used to make that product” but it was expensive, time consuming, and "very high-end chemistry.” Most labs were doing cannabis analysis “in a very prescribed way,” she mentioned, and couldn’t “just, all of a sudden, change up our chemistry to see these molecules” (audio - 3m).
- Gang brought up the limits of analysis, remarking that everyone agreed “safety’s important” but setting public policy and standards for “what is a safe mixture, what is the safe product” remained difficult. When it came to effective lab standards for detection of byproducts, he felt the technology “works, but it’s also very limited in what it can tell you.” Gang offered the analogy of “a television set from the 1970s” where the image resolution was far lower, and looked fine watching older broadcasts. He compared content filmed for more modern high definition televisions which would lose significant detail if viewed on older equipment, “and that’s kind of like the technology that we’re dealing with here, too.” To examine cannabinoid samples with better detail, labs needed better instrumentation and methods, Gang reported, or “there may be other things in there that you just cannot see” which had health implications (audio - 7m).
- Tonani found we "end up with a lot of known unknowns” because testing standards were not yet uniform, and “the more detailed the chemistry gets, the harder it is for us to actually have standards.”
- Wise called attention to the fact that there were also many tests aside from those for cannabinoids, including metals tests, residual solvent tests, or detecting other molecules like phytochemicals. The evaluations of cannabis plant chemistry were “almost endless.”
- Douglass suggested an "ingredient standard" could be specified by officials to determine what could be within an ingredient. Ingredient standards required substances meet a defined purity level as well as “a certain level of residual solvents” or limits on byproducts present, and overall was a "pragmatic way" to address this issue by giving “reasonable certainty about the things we don’t want included or that present a potential safety issue.”
- Stella revisited “potential toxicity” of the compounds, stating that while “moderately high” levels of delta-9-THC had been present in older cannabis samples, cannabis concentrates led to “new questions about the safety profile” at higher THC concentrations. With CBD, based solely on “public experiment,” the compound appeared to be “quite safe” with not "too many horror stories." Stella had seen “artificial” cannabinoid molecules like Spice or K2 could be highly toxic in large doses and that those consuming them had been reported in the hospital with renal failure “or seizures.” Dosing and toxicity concerns were present with delta-8-THC, he commented, and “until we actually do” testing scientists couldn’t say how “individuals are responding to this new molecule” (audio - 4m).
- Wise described the conversion of CBD into types of THC. “There are different steps along the pathway,” she said, including the most economical and “highly used transformation at the moment which is, you know, converting CBD into delta-8” (audio - 6m):
- Question 3 -“What amount of commercially available (produced and sold) delta-8 THC is produced from cannabidiol (CBD) relative to the amount that is simply extracted from the cannabis plant?” (audio - <1m)
- Douglass believed it was “impossible to know all the details” of delta-8-THC in products already available in the market, but shared some related “economic information” (audio - 3m).
- For CBD converted into delta-8-THC, he said the starting material was “less than $500 a kilo[gram]” and along with a few reagents someone could convert that into delta-8-THC for around “a cost less than a thousand dollars a kilo.”
- Since delta-8-THC seemed to appear naturally in minute and potentially undetectable levels in cannabis and hemp plants, Douglass stated that extracting the compound directly from plants meant “the cost of production is significantly more.” For this reason, he suspected “most, if not all" delta-8-THC in products was converted from hemp-derived CBD.
- Tonani added the point that hemp was generally “much lower priced to produce” in part because it was “substantially less regulated.” Douglass agreed the prevalence of delta-8-THC had “mushroomed recently” which could be attributed to hemp oversupply.
- Gang asked whether plant-produced delta-8-THC was “a pure form,” or a “mixture” with other enantiomers. Douglass replied that the conversion chemistry involved "at least a ring closure" since CBD lacked “stereogenic centers” and the process created them. While Douglass didn't have data on the stereoselectivity of delta-8-THC “produced from CBD,” he was confident that a mixture of compounds was likely since he hadn’t yet seen procedures for producing pure enantiomers. He added that when producing delta-8-THC from delta-9-THC, the “isomerization process can scramble stereochemistry” (audio - 2m).
- Tonani said selective breeding of cannabis plants may produce greater delta-8-THC but researchers remained unsure about its provenance. It was possible the molecule was made through a naturally occurring isomerization of delta-9-THC, she said. Douglass agreed that delta-8-THC had been found “on” cannabis plant samples but it wasn’t known if the plant was making the compound or if it was a result of THC acid “drying or processing.” Gang noted there were standards for analyzing delta-8-THC in hemp, but the molecules had never been found to his knowledge. THC levels in hemp were “low in general” and if the plant was making it, then it “may just be way below a level that’s detectable” (audio - 3m).
- Poolman asked if any licensed cannabis processors had been extracting delta-8-THC from cannabis. Tonani remarked that the compound had “never” been observed in cannabis flower at high enough levels. Douglass agreed and said when delta-8-THC was found in samples, it was at around 0.01% of tested plant material and therefore not economical to extract. He stated there were “only a few instances where delta-8 has been reported in the literature as being found in cannabis plant samples” and always “in much smaller quantities” (audio - 4m).
- There are multiple patents registered for the conversion of CBD into THC compounds.
- Douglass believed it was “impossible to know all the details” of delta-8-THC in products already available in the market, but shared some related “economic information” (audio - 3m).
- Question 4 -‘From a chemists perspective, what does “synthetic” mean? How does this differ from “artificial”?’ (audio - <1m)
- Gang said artificial meant a “completely new" molecule with a “similar effect” that binds “to the cannabinoid receptor but they’re not related at all structurally to the phytocannabinoids...completely different than what’s found in nature.” Synthetic molecules, he said, were lab-made but were “exactly the same molecule.” Gang used the example of sucrose---sugar---which could be extracted from sugar cane or produced synthetically with petroleum, as compared with Splenda, an artificial sweetener and completely different substance with some chemical properties similar to sugar. For cannabinoids, he said the options were between the “same molecule that’s found in the plant” but created in a lab, and “something that binds to the receptor and has a similar kind of function in the body” while still “a completely different molecule that was made up in the lab from scratch” such as Spice or K2 (audio - 3m).
- Stella asked about enantiomers, or differences between delta-8-THC produced by plants versus when the compound was synthesized. Gang responded that “nobody’s really looked,” leaving it unclear whether delta-8-THC is produced by cannabis plants. He nonetheless found it “very, very unlikely” that not-yet-identified enzymes in cannabis were producing delta-8-THC because after looking at the cannabis genome “there’s nothing that looks like that” (audio - 2m).
- Douglass mentioned food sector use of terms like "nature identical" or "lab grown" to describe molecules synthetically produced that are not artificial, asking for Gang’s opinion of the practice. Gang noted that citric acid was a common food additive first identified in citrus fruits but that “we don’t get it from citrus fruits anymore, it’s all made in vats by yeast in a lab.” This process was more affordable and produced “the same exact molecule,” he added. Phytocannabinoids, Gang said, were "restricted to a very limited set of plants,” to date only in cannabis, and that “other plants do not make these molecules” though people had looked for their occurrence (audio - 2m).
- An October 2020 Trends in Plant Science review noted that phytocannabinoids “occur in flowering plants, liverworts, and fungi,” though they had been “first isolated from Cannabis sativa.”
- Poolman asked whether delta-8-THC counted as a phytocannabinoid, and Gang answered that it was, in addition to CBD, since the molecules were similar and “put together from the same general building blocks.” Not all phytocannabinoids could bind to cannabinoid receptors, he said, and differed from endocannabinoids which humans and some animals naturally produced (audio - 6m).
- Stella explained that known phytocannabinoids had been estimated at around 60 varieties but “now we’ve moved on to a hundred phytocannabinoids.” However, the majority of them didn’t cause biological responses, he indicated. Besides THC and CBD, Stella identified cannabigerol (CBG) and cannabidivarin (CBDV) as other compounds which provoked some biological response from the body. He commented that dosage was important in evaluating the body’s response to a molecule as “everything taken at very high dose becomes toxic, it’s all about moderation.” Stella described how the endocannabinoid system worked “to control pain, and those phytocannabinoids...hijack this system.”
- Responding to a public comment in the chat box, Douglass reported that the statutory definition for synthetic cannabinoids in Washington was “more aptly described as artificial cannabinoids.” He said substances like spice or K2 were artificial, not synthetic cannabinoids but “there’s history there why they’re called synthetic” (audio - 1m).
- Question 5 - “Are temperature changes and solvent-use unique to extraction and processing of cannabis or are they used for other extracting and processing other plants?” (audio - 1m)
- Douglass answered that “temperature changes and solvent use are not unique to extraction or processing of cannabis” and that such processes were “used ubiquitously throughout the plant and food processing world.” He noted temperature changes were part of pasteurization and that all “natural flavors we have in commerce” have been extracted by “some solvent” and that “certain manufacturing functions...are applied to cannabis [and] are found all over the place.” Douglass pointed out that applying heat to cannabis can “take your cannabinoid acids and decarboxylate those to your neutral cannabinoids” but said both temperature and solvents were part of the chemistry for other food processes “everywhere.” Gang readily concurred (audio - 4m).
- Question 1 -“How do the effects of delta-8 THC differ from those of delta-9 THC (and other THC isomers)? Does it matter if the compound is produced in plants or chemically?” (audio - <1m)
- Panelists and staff fielded an array of questions from attendees about the science of the plants and the policies around their regulation.
- Michael Goodman asked about Hoffman’s post graduate degree (audio - 1m).
- Brandon Jeffery wanted to know about stereochemistry concerns and whether stereochemistry in cannabinoid molecules was “unlikely to affect the user experience or safety” (audio - 5m).
- Douglass replied that it wasn’t yet known though there were “some indications that they might proceed through similar metabolic routes, but we’re unsure at this stage.” Gang termed the molecules “relatively complex” and that the “right and left-handed versions tend to have different qualities” and that it would be “very unusual for the molecules that have very different shapes” to produce the same effect in the body. Stella agreed, saying it was “always also a question of dose” when toxicity came into play for an ingested substance, but given funding it would be possible for more studies to be done “quickly.”
- Rian Takahashi, United Western Green LLC, wanted to know what agency staff would do to protect the cannabis industry producers who collected cannabis biomass for extraction. Hodgson noted that was a policy question and part of why WSLCB staff convened the deliberative dialogue. Hoffman added that since the event focused on plant chemistry they’d address Takahashi’s policy concern at a later time (audio - 2m).
- Jeff Wilhoit, Puffin Farm, inquired about safe methods used for creating delta-8-THC, or processes that used “LCB approved solvents.” Wise couldn’t speak to what would be considered safe or healthy, but thought using approved solvents was "potentially possible,” such as a “liquid-liquid wash." She felt that employee safety and cleanup steps in converting cannabinoids was more important than “the synthetic route to get there” (audio - 2m).
- Rusty Sutterlin, Inventure Renewables Inc. CSO, asked about decarboxylation and whether it was “considered a synthesis” method. Douglass responded that panelists had discussed if there was “a dividing line between ‘chemically altered’ and something that’s the product of synthesis” and that both processes existed in commercial food production where “you are doing chemistry.” However, the consensus emerged that decarboxylation wasn’t the same as synthesis though there were challenges in defining it, he told the group. Tonani added that the “science isn't, you know, black and white” and created regulatory challenges. “There’s no bright line answer here” even for scientists, Douglass said (audio - 3m).
- Claire Stenersen, SōRSE Technology Associate Scientist, wondered about the natural enantiomer for THC in cannabis plants and whether other enantiomers were classified as “artificial.” Gang answered that only one enantiomer had been observed in nature and unless a compound’s enantiomer was present in nature it “would be considered artificial.” Douglass included that there were “different stereochemistry concerns” for different cannabinoids (audio - 3m).
- Gregory Foster, Cannabis Observer Founder, returned discussion to the topic of synthetic cannabinoid law, saying the distinction between synthetic and artificial provided by the panel made it appear that a law on synthetic cannabinoids was actually addressing artificial cannabinoids (audio - 5m).
- Gang indicated that it was possible to make synthetic delta-9-THC, but his understanding of the law worded it as cannabinoids “being extracted from the cannabis sativa plant.” He speculated it had been possible for individuals to “skirt the law” by having identical cannabinoid compounds synthesized and not derived from cannabis plants, “and the law was written in a way so that they can’t do that. That’s why ‘synthetic’ is used in the law, from my understanding.”
- Stella mentioned that “the definition of cannabinoids [is] two-fold, and that’s, I think, where the challenge is.” His understanding was the law intended to prohibit cannabinoids produced by the plant in addition to cannabinoids “added to that definition and those are” artificial “but because they produce psychotropic effects they have the activity of cannabinoids.” He identified federal drug scheduling as including cannabinoids “as an ensemble” of artificial and synthetic compounds defined by their “structure” rather than their source.
- Tonani pointed out that a synthetic THC, Dronabinol, was a schedule III substance while most cannabinoids and the plant were schedule I which led to “very different tracks that have kind of all collided, making it really complicated.”
- Brooke Davies, Washington CannaBusiness Association (WACA) Deputy Director and Associate Lobbyist, asked about cannabis testing lab capabilities and the analogy offered of older analogue versus high definition television. She was curious whether the limitations in testing specificity “exist currently on the adult use market” for cannabis plants from licensed producers, and if “our lab technology [needs] to be updated broadly to make sure that all the products are safe?” (audio - 7m)
- Wise believed existing lab instrumentation was "perfectly fine" for what it was required to test for. However, when individuals contacted labs about purchased cannabis to see “if it’s safe or not,” she always told them lab staff couldn’t answer that question as “that is not a test we run,” they only looked for a list of compounds at certain levels of detection. While new testing could be developed for the instrumentation her lab already had, Wise made clear that it took “tens of thousands of dollars to see a new molecule” and otherwise, “if we're not looking for it, we're not going to see it.” This meant it was possible substances not safe for consumption could be in consumer products without ever having been identified, she explained, though “if it was dangerously dangerous we would see, you know, public health problems.” Wise used the example of some metals, saying “we’re only supposed to look for four, but not even in Washington state in all products.”
- Tonani agreed some cannabinoids were tested for, while others weren’t, even as the composition of cannabinoids differed among the cultivars of the cannabis plant.
- Douglass elaborated that there would always be “unknowns” from a scientific testing standpoint regardless of whether compounds were artificial or synthetic, and that more money for equipment and research would always yield new insight. He asked, “How much do you want to pay to know a larger fraction, or percentage, of those unknowns?”
- Gang didn’t think testing instruments were “antiquated and totally useless” but wanted to convey that normal testing for cannabis wouldn’t list “everything that's in that sample.”
- Sutterlin inquired if compounds could be described as natural provided “the starting material is from nature,” offering examples like wood pulp. Gang said that was “a regulatory question, how it’s defined is not an actual scientific question” but the U.S. Department of Agriculture (USDA) and the Food and Drug Administration (FDA) typically set policies around product claims. Douglass agreed, saying regulations varied around the world (audio - 2m) .
- Learn more from the FDA page on Use of the Term Natural on Food Labeling as well as the Federal Trade Commission (FTC) consumer protection article titled Are your “all natural” claims all accurate?
- Sutterlin also asked about the negative health effects “related to the stereochemistry of delta-8.” While he had personally not heard any problems about it, due to the lack of research and the “huge amount of delta-8 out on the market,” he wanted to know if panelists were aware of anything beyond anecdotal evidence (audio - 6m).
- Tonani had not heard of problems related to stereochemistry, but was aware of “increases in poison control and things like that” although she’d seen no peer-reviewed data on problems.
- At publication time, Cannabis Observer was not able to identify mention of delta-8-THC on the Washington Poison Center (WAPC) website.
- Douglass called the prominence of delta-8-THC “too recent” and no one had separated what reported effects were “related to chemistry, and what are related to mixtures of compounds being called delta-8-THC.”
- Stella mentioned that most situations of product toxicity were only identified in “the emergency room.” He suggested delta-8-THC hadn’t been deeply studied "because it's not that exciting for scientists" compared to the chemically similar delta-9-THC.
- “Teasing out if there’s an issue,” with health effects of delta-8-THC reminded Tonani of the "vape cartridge issue" where it had taken “quite a while to kind of get the samples and figure out what, you know, was a causative agent.” She described how there might be “other things outside of delta-8 that are in the product” or “the stereochemistry of the delta-8 itself” and experts needed to “tease out” what was “being seen out there.”
- Douglass repeated his recommendation of an “ingredient standard,” suggesting implementation of such a standard meant some possible risks “could be addressed proactively.” He suggested possibly only allowing one enantiomer as well as prohibiting “certain levels of potential byproducts.” As a scientist, he believed there was “a path to addressing it that way...that doesn’t rely on us waiting and seeing” effects in hospitals. Stella agreed, “I think we have the tools to be proactive,” he said, “and catch up” to a compound already being consumed by the public. Tonani also agreed that the industry and officials could define “a quality standard that products have to meet.”
- Tonani had not heard of problems related to stereochemistry, but was aware of “increases in poison control and things like that” although she’d seen no peer-reviewed data on problems.
- Blade Bolden, Unicorn Brands LLC General Manager, inquired if there was a “distinction between a solvent being used for post-processing or purification versus a solvent that is used for extraction specifically?” Douglass viewed that as a regulatory question, but emphasized that from a scientific perspective, “it doesn't matter where along a process a solvent is perhaps being used...as long as it's not in the end product, or there in levels low enough so that it’s not a concern” (audio - 3m).
- Dan Oliver, ACO Clear Director of Development, wondered about the conversion of CBD to delta-9-THC, and if there was “any reason why that shouldn't also be as concerning” as the conversion to delta-8-THC (audio - 5m).
- Wise confirmed it was “possible” to convert CBD into delta-9-THC and that “all the same [process] concerns we’ve raised are also valid.” If there was “a very highly functioning traceability system in this state it would be possible to utilize CBD from plants that were tagged” through traceability to be converted into delta-9-THC, she said, but for a person to do that when they’re licensed to grow plants for delta-9-THC specifically “seems silly to me.” As to the issue of “CBD coming from outside of Washington state” which was converted into delta-9-THC “and potentially introducing that into sale in Washington,” Wise understood that to be “not legal under the current system.” However, she had “no concept of how prevalent that process might be at this point.”
- Douglass promised he could “make you pretty much” any compound given a few elements and “enough time and resources.” Stella said a research paper had claimed orally administered CBD could convert to delta-9-THC during digestion, but that the article was incorrect and had been “retracted, and confirmed that it was wrong.” He affirmed that there was “no known way” CBD could transform into delta-9-THC within the body. Douglass stated that it was simpler to convert CBD into delta-8-THC as the “chemistry to do so is less selective."
- Lukas Hunter, Harmony Farms Director of Compliance, wanted to know more about the distinction “between extraction and refinement” and how much panelists thought the distinction mattered. “Is it always extraction,” he asked, “or is extraction the initial point of taking biomass and converting it into a[n] oil that needs further refinement?” Gang found that this could be a policy question based on what regulators chose to “define as the extraction.” But from a “natural products chemist’s perspective,” extraction was “pulling a molecule out of the plant material” and anything past that stage, “we’re then going to purify it.” He cautioned that lawmakers or agency leaders might define some or all purification processes as part of cannabis “extraction” (audio - 4m).
- Jim MacRae, Straight Line Analytics, wanted to know what panelists thought about the “equation of THC with potency in current law.” Wise replied that “the word ‘potency’ is never used correctly in the cannabis space” when referring to the concentration of “cannabinoids in a mixture.” While this use appeared in “policy regulation language,” she wished it would become less common (audio - 3m).
- In pharmaceuticals, potency meant “the amount of an active molecule that your body actually uptakes and processes,” Wise relayed. Whereas “what we call potency in the cannabis space is actually a percent by weight, generally, of cannabinoids.”
- She added that the “psychotropic nature of other molecules is yet to be fully determined scientifically.” Psychoactive can “just mean it’s acting on your brain, caffeine is psychoactive, CBD is psychoactive” because those substances enact “changes or interacts with your brain.” Wise described any compound that “alters your perception of reality” as psychotropic, and to strictly equate “potency with amounts of THC” was potentially overlooking the “psychotropic nature” of other molecules found in the cannabis plant.
- Jeremy Moberg, CannaSol Farms Owner and Washington SunGrowers Industry Association (WSIA) Board Member, brought up banning delta-8-THC from the legal market, which he had previouslyadvocated for: “Given the lack of understanding of the science and purity should the state allow synthetic production as it currently does?” Moberg questioned the appropriateness of discussing the science “with so many unknowns at the same time that this product is already available” instead of completely studying the ingredients “before we were letting this out and to consumers” (audio - 7m).
- Douglass recognized the concerns around the processes for converting cannabinoids, “but I do see a path with ingredient standards and the proper regulations to test and regulate these products” for their safe use.
- Tonani concurred, even though “it's not going to be an easy path” with lingering questions like the science and “impact to other stakeholders...but I do see a path on how you could regulate it.” Being “brutally honest,” she said, as a medical cannabis patient for over 20 years, she’d often seen the cannabis sector “jumped off” ahead of the science, and “this time we’re trying to kind of invert that.”
- Wise argued that “the crux of some of this” was the statutory spaghetti of “state regulated, high-THC” cannabis in Washington “versus federally quasi-legal CBD/hemp” in addition to in-state hemp “all regulated by different bodies, they all have different rules.” She believed a single federal standard would have helped the state to avoid “many of these situations.”
- Douglass posited that "the enterprise of science is fraught with unknowns" but people unfamiliar with the discipline may view it as “exact and specific.” He believed that if society waited to completely understand the safety of a substance like cannabis “we wouldn’t have regulated systems at all.” As a parent, Douglass would rather his child obtain delta-8-THC products “from a tested and regulated system” than an unregulated one.
- Looking at delta-9-THC and CBD products, Stella suggested experts had “some understanding” of the compounds’ effects on the body and so products were “doing pretty well.” But delta-8-THC wasn’t as well understood, and he claimed pharmacologists wanted "baby steps" of making low dosage and limited products available until “we have the data...maybe starting slow would be the way to go.”
- Bonny Jo Peterson, Industrial Hemp Association of Washington Executive Director, asked about the potential for research collaboration, and how could “the cannabis industries collaborate to have the needed research conducted?” (audio - 10m)
- Tonani emphasized the need for research funding, which was often from government bodies or the private sector. With cannabis, funding from “government agencies has been seriously lacking and seriously restricted,” she said, noting a research project at the Lambert Initiative for Cannabinoid Therapeutics at the University of Sydney had a significant private endowment. There were also restrictions due to the federal prohibition on cannabis that impacted research and “arbitrary regulation around THC content that makes research difficult,” Tonani stated. But she considered collaborating on funding to be the top way the cannabis sector might contribute to the issue. Tonani also indicated some research issues like testing for the presence of metals might be conducted on either low-THC hemp plants or higher-THC cannabis plants exclusively, but that the results would have applicability to “both plant types.” She recommended lobbying government agencies, research institutes, and private organizations to invest in cannabis research.
- Stella joined the call for more research funding. While there was federal funding with “very specific types of questions” those officials were interested in, “parallel” state level research did not support efforts such as “researching the products that are currently available in Washington.” Dual tracks for research would be “very important,” he reasoned, as Washington topics would differ from federal investments.
- Learn about the cannabis research money approved in the state’s 2021-23 fiscal biennium operating budget that was signed into law on May 18th, specifically:
- UW: $626,000 “for cannabis research at the university to collaborate with the Washington State University collaboration on cannabis policy, research, and outreach [CCPRO] to create frameworks for future studies.”
- WSU: $100,000
- Washington State Board for Community and Technical Colleges (WA BCTC): $100,000 “to collaborate with the Washington State University collaboration on cannabis policy, research, and outreach to create frameworks for future studies.”
- The Evergreen State College (TESC): $75,000 “solely for the institute to review available research literature to investigate and describe any relationship between early substance abuse of cannabis, opioids, or cocaine and mental health disorders in young adults.”
- Washington State Health Care Authority (WA HCA) - Community Behavioral Health Program: $500,000 “solely for the authority to contract on a one-time basis with the University of Washington alcohol and drug abuse institute to develop policy solutions in response to the public health challenges of high tetrahydrocannabinol potency cannabis.”
- Wise agreed about the expense of research, noting previously introduced legislation to establish a cannabis commodity commission in Washington state would be capable of funding some projects. “Many, many industries, agricultural industries, have structures like this,” she remarked, “where there is fundamental research that gets done.” Wise wanted a system that would “transcend” the regulatory differences between hemp and cannabis plants because “we’re talking about more-or-less the same plant here. It is the same plant, scientifically.” Tonani agreed “only THC” differentiated cannabis and hemp, and “99.9% of the things that are, like, interesting for research perspective are exactly the same.”
- According to Gang, the existing federal policies for cannabis meant that WSU or UW “have to be careful in certain types of research that they can do...we can’t grow high-THC plants. We’re just not allowed to.” He stated that as public universities received significant funding from the federal government as well as the state, they had to observe the restrictions on what manner of research they could engage in. Hemp was a “completely different situation,” Gang said, and the state could continue to “define how these plants are viewed” and distinguish the “research perspective in maybe a slightly different way than we do from a consumer perspective.”
- Hoffman and Nordhorn thanked the panelists for their participation, and Nordhorn asked a couple of final questions:
- Phytocannabinoids vs. Synthetic Cannabinoids (audio - 4m). Nordhorn wanted clarity “as a lay person and non-scientist” if a synthetic cannabinoid that was identical to a phytocannabinoid was considered to be a phytocannabinoid, “or is that just classified more as a synthetic itself?” Gang said if it was the “exact molecule” as a phytocannabinoid in the plant, “it's still the same class of compound” even if it was created in a laboratory. Nordhorn then asked whether an artificial compound would ever be a phytocannabinoid, to which Gang replied “yes and no” because if an artificial compound had the “general structure” of a phytocannabinoid as the class of compounds used by chemists one could “still call it the class of a phytocannabinoid, from a chemist’s perspective.” Douglass said a cannabinoid compound matching one in the plant was a phytocannabinoid “irrespective of the source,” with Tonani adding any difference between a naturally occurring and synthetic compound couldn’t be differentiated by an existing “analytical method.”
- CBD Conversion to delta-9-THC (audio - 1m). Nordhorn also asked Douglass about his statements on the difference between converting CBD into delta-8-THC versus delta-9-THC, wanting to know why the latter conversion was more difficult. Douglass explained that delta-8-THC was “more stable, thermodynamically” and CBD compounds would “rather be delta-8 than delta-9, energetically.”
- Hoffman offered her appreciation to all participants for the “great discussion,” and predicted staff would follow up with panelists “in the not so distant future” with new questions. After staff had a chance to “curate” what they’d heard she assured stakeholders that agency leaders remained committed to “an interactive process” of rule and policy development. Hoffman was aware of the “variety of interests involved around this issue” and anticipated that staff would not have any updates for “the next couple of weeks” (audio - 2m).
- As regards the THC rulemaking project, Hoffman previously stated the tentative timeline would have a public comment period run until Friday July 2nd with a possible rule proposal by August 18th. Under this timeline, she indicated a public hearing would be held on September 29th.
- During the June 8th board caucus, board members talked about inviting the panelists to hear from them directly.